Pediatric Anesthesiology 2013
a Win for SPA
WOW! The Las Vegas Winter SPA Meeting was a total WIN for the SPA, with more than 800 attendees, wonderful plenary presentations, and lots of opportunity at hands-on workshops. In an unusual turn of Fate, we even had near-perfect, non-SPA-like weather, too!
One of the hottest tickets in town was for the brand-new Young Turks reception, an opportunity for SPA leadership to meet and greet, connect faces to names, with some of our outstanding young members. How FUN for us to meet those who will, undoubtedly, be running your Society in the next ten years or so. Our beloved Myron Yaster deserves all the credit for this fabulous idea, and for bringing his enthusiasm and his (huge) Heart for Mentoring to the party. Thanks, Myron! But do not despair—we are already PLANNING to have another Young Turks gathering at the 2014 Winter Meeting, so that your Officers and Board can meet even more of our young, talented members. The Winter Meeting next year will be in Fort Lauderdale, FL, a chance to pretend that it’s Spring Break all over again!
Another huge development for the SPA was the successful “spawning” of the Society for Pediatric Pain Medicine (SPPM) as a separate Section, akin to the Congenital Cardiac Anesthesia Society (CCAS). With Section status, the group will have its own budget, as well as mechanisms for including non-physicians and non-anesthesiologists for membership and speaking roles. A Hearty Congratulations and Thanks are due to Sabine Kost-Byerly and her colleagues for their efforts to organize the Section.
Furthering our educational mission, the SPA is sponsoring two identical weekend courses: “Intensive Review of Pediatric Anesthesia” focusing on core concepts in our specialty, aimed at both generalists and specialists. One was held May 17 – 19th in Dallas, Texas; the other will be held August 23-25th, in Chicago, IL. Both courses sold out early, but we were able to add capacity at both locations, nearly doubling the size of the courses. Please follow this link for more information: http://www.pedsanesthesia.org/meetings/
This fall, the SPA will join with the European Society for Paediatric Anaesthesiology to present a joint meeting in Geneva, Switzerland, September 5-7, 2013, to be held at the headquarters of the World Health Organization. Not only is this a fabulous venue in the center of Europe, but the program promises to deliver strong content emphasizing our common interests and variations in style of practice. Cycling and wine tours, a tour of the accelerator at CERN, and opportunities to mingle with colleagues from across Europe make THIS meeting a perfect beginning or end to a European vacation. Check out this link for details and registration: http://www.euroespa.org/espa-congress-calendar.html
Last but certainly not least, it’s not too early to plan your trip to San Francisco for the SPA Annual Meeting, to be held Friday, October 11, 2013, just before the ASA Annual Meeting begins. This year’s theme is on Neonatal Anesthesia. My personal mantra #1 is “never trust anyone under 10 kg,” so I’m hoping to learn some new tricks for staying out of trouble! Please plan to join us in San Francisco.
Your Society is busy, active, and needs YOUR input and participation. Strong efforts in Quality and Safety, Education, International Service and Education, coupled with new initiatives in research and member communication make the Society for Pediatric Anesthesia THE VOICE for Pediatric Anesthesiologists in the USA. I welcome your suggestions, criticisms, and engagement as your Board works to represent YOU.
Meeting Reviews and Checklists
If, like me, you were unable to attend the immediate past SPA meeting held in Las Vegas this past March, then this newsletter is definitely for you. A cohort of faithful SPA members reviewed the meeting in detail and their reports form the bulk of this newsletter. Of course, for those that did attend, information overload may have been an issue or you may have missed a session due to other obligations. These reviews are for you too!
Everyone should be sure to read the article detailing the work of SPA’s Quality and Safety Committee that has resulted in the development of the Critical Events Checklists for use in the OR. These checklists are freely available through the SPA website and the link is provided within the article.
It remains for me to wish all SPA members a wonderful summer and I hope that busy ORs don’t prevent everyone from spending some fun time in the sun with family and friends.
SPA launches Pediatric Critical Events Checklists
By J. Nick Pratap, MA, MB BChir, MRCPCH, FRCA
Cincinnati Children's Hospital Medical Center
Cincinnati, OH
and Erin Pukenas, MD, FAAP
Cooper Medical School of Rowan University
Camden, NJ
The blood pressure rises to 160/95 shortly after induction. Your 7-month old patient has already received more than enough muscle relaxant. Administering a large bolus of fentanyl does nothing. Even an attempt to deepen anesthesia fails to control the BP’s upward trend. As you now glance at the ECG tracing on the monitor, you notice that the ST segments are starting to sag downwards. With an inward sigh, you realize this is not going to be a routine circumcision…
Pediatric anesthesiologists are no strangers to managing emergency situations. Of course, experienced attendings manage uncomplicated laryngospasm or mild bradycardia, for example, on a routine basis without breaking a sweat. Certain crises however arise so infrequently that individual practitioners experience them personally only a few times within their professional lifetimes. Under such circumstances it can be hard to recall full details of all necessary facts, decision points and management steps.
Refresher lectures, departmental review meetings, case report articles and conference presentations all help keep us up to date. But do you always feel fully equipped to deliver the very best evidence-based practice every time something unusual takes place and requires urgent action?
The Society for Pediatric Anesthesia (SPA) Quality and Safety Committee has recently completed a two-year project to address just these challenges. Inspired by ultra-safe industries like aviation and nuclear power generation, committee chair Genie Heitmiller and colleagues have created a compendium of crisis checklists for consultation in the pediatric OR at the first hint of trouble.

The committee chose 17 challenging emergency problems for this treatment. Members divided the list between institutions from across the nation. Within their departments small groups collaborated to create one- or sometimes two-page bullet-pointed checklists. Each card starts by summarizing the problem it addresses, and then lists relevant factors to consider and possible steps to take in the event of that particular emergency. The checklists include drugs treatments if appropriate, along with a handy dosing reference.
Every checklist was circulated amongst the whole committee. Each was then sent out to a different institution for peer review and, in some cases, road-testing in high-fidelity simulation. Dr. Heitmiller worked diligently to coordinate all feedback and revise the cards accordingly.
The full list of topics covered by the checklists is:
- air embolism
- anaphylaxis
- bradycardia
- cardiac arrest
- difficult airway
- fire (airway and OR)
- hyperkalemia
- hypertension
- hypotension
- hypoxia
- local anesthetic toxicity
- loss of evoked potentials
- malignant hyperthermia
- myocardial ischemia
- tachycardia
- transfusion and reactions
- trauma
Not only has the content of the emergency reference cards been subject to thorough deliberation, but their design has also been carefully reviewed. NASA research on checklist usability, as well as review by human factors experts, contributed to the eventual format. Our profession’s unique requirements were kept in mind throughout this process. For example, the committee considered that checklists will often be read with the lights dimmed for laparoscopic surgery. Furthermore, the average age of members’ eyes may be a little higher than those of the jet fighter pilots that NASA targeted, so small print was avoided. The committee recognized that the checklists need to be useable in a multi-professional environment, and also that they may be presented in the OR in either hard copy or electronic format, according to institutional preference.
An additional consideration in the presentation of the checklists stemmed from an independent but similar project led by Atul Gawande, Harvard surgeon and celebrated author of The Checklist Manifesto1, as well as other books and articles. Gawande’s group has also created a set of emergency crisis checklists, but theirs are more general in scope, and omit many of the scenarios previously identified by SPA’s Quality and Safety Committee for their particular pediatric relevance. Given that many society members also take care of adult patients, and will therefore be exposed to that initiative too, the SPA group settled on a format to complement the Harvard group’s work.
The Harvard group has proceeded to demonstrate superior management of emergency situations in a high-fidelity simulation environment. This important finding bodes well for the impact on patient care of SPA’s checklists too. Published in the New England Journal of Medicine in January 2013, their study simulated 106 OR crises. 17 multidisciplinary OR teams, each including anesthesia providers, did their best to manage these time-critical scenarios to successful outcome. The key result was that teams randomized to use a checklist missed only 6% of previously identified crucial steps, as compared to 23% omitted by teams without access to the checklists (p<0.001). A further validation of the checklist approach came from questioning members of the teams who had used the checklists. 97% stated that they would want the checklists to be available to providers if they themselves were patients undergoing surgery.
The SPA Quality and Safety Committee would like to thank the many institutions and pediatric anesthesiologists who contributed to the crisis checklist project, including the following:
- Arkansas Children’s (Jay Deshpande)
- Beth Israel Deaconess (Athina Kakavouli)
- Chicago Children's (Butch Uejima)
- Children’s Hospital of Philadelphia (Jeff Feldman, Don Tyler)
- Children’s National Medical Center (Jamie Schwartz)
- Cincinnati Children’s (Nick Pratap, Jim Spaeth, Anna Varughese)
- Columbia-Morgan Stanley (Susan Lei, Susumu Ohkawa, Lena Sun)
- Cooper Medical School of Rowan University (Erin Pukenas)
- Emory (Steve Tosone)
- Johns Hopkins (Genie Heitmiller, Hal Shaffner, Myron Yaster)
- Mott Children’s (Shobha Malviya)
- Nationwide Children’s (Tom Taghon)
- Nemours duPont (Randy Brenn)
- OHSU Doernbecher Children’s (Dan Woodward)
- Pittsburgh Children’s (Jerry Parness)
- St Louis Children’s (Jim Fehr)
- Seattle Children’s (Lynn Martin, Jai Rampersad, Sally Rampersad)
- Texas Children’s (Kalyani Govindan, Imelda Tjia)
- Vanderbilt (Scott Watkins, Gina Whitney)
We would also like to thank the SPA Executive Board for their support and approval of this project, as well as Kim Battle and Stewart Hinckley at the SPA administrative offices (Ruggles).
SPA’s Pediatric Critical Events Checklists are freely available on the SPA website at http://www.pedsanesthesia.org/newnews/Critical_Event_Checklists.pdf.
SPA’s intention is that this webpage reference will always connect to the latest version of the checklists, so it may be bookmarked on your computer or smartphone for reference in an emergency.
References:
- Gawande A. The checklist manifesto: how to get things right. New York: Metropolitan Books, 2010.
- Arriaga AF, Bader AM, Wong JM, et al. Simulation-Based Trial of Surgical-Crisis Checklists. N Engl J Med 2013; 369: 245-53.
Pediatric Anesthesiology 2013 Meeting Reviews
Thursday, March 14 - CCAS Morning Sessions
By Wilson Chimbira, MD, FRCA
University of Michigan Mott’s Children’s Hospital
 The Annual Meeting of the CCAS was introduced by new President Dr. Helen M. Holtby (Hospital for Sick Children) who gave the opening remarks. Dr. Jumbo Williams (Stanford University) and Dr. Wanda Miller-Hance (Texas Children's Hospital) and the rest of the planning committee should be commended for once again putting on an excellent program.
The Annual Meeting of the CCAS was introduced by new President Dr. Helen M. Holtby (Hospital for Sick Children) who gave the opening remarks. Dr. Jumbo Williams (Stanford University) and Dr. Wanda Miller-Hance (Texas Children's Hospital) and the rest of the planning committee should be commended for once again putting on an excellent program.
The first session of the CCAS meeting was titled Basic Science and Practical Application: Cardiopulmonary Bypass (CPB) and was moderated by Dr Scott Walker (Riley Hospital for Children). The first speaker was the outgoing president of CCAS, Dr. James A. DiNardo (Boston Children’s Hospital). He started off the session talking about the physiologic trespass of cardiopulmonary bypass in a talk titled ‘What’s so bad about Cardiopulmonary Bypass – We do it Every Day?’ He broke down the effects into four different categories. Firstly, there is the inadequate suppression of thrombin and this occurs despite using high doses of unfractionated heparin. He also stressed that the ACT is an inadequate measure of thrombin suppression. The thrombin generated during cardiac surgery plays an essential role in platelet activation, production of cytokines and prostaglandins. Secondly, he highlighted that there is an inadequate suppression of inflammation during CPB. The inflammatory process is complex and involves multiple interconnected pathways so it is hard for any single countermeasure to suppress it. Thirdly, he mentioned hemolysis with the consequent development of increased levels of plasma free hemoglobin and urinary free hemoglobin. Elevated levels of free hemoglobin can induce direct renal injury and are import causes of CPB induced end-organ dysfunction. Finally, he turned his attention to oxygen delivery (DO2) and the microcirculation during CPB, reminding us of how poor our understanding of this is and how inadequate DO2 may lead to end organ dysfunction.
The next talk was by perfusionist Colleen Gruenwald (Hospital for Sick Children) titled ‘Cardiopulmonary Bypass: Current State of the Art and New Development’. This talked focused mainly on the development of bloodless pediatric heart surgery. She opened up by summarizing that this can only be achieved by a team effort including the family, cardiology, surgery, anesthesia, perfusion, ICU and the laboratory. The patient selection process is important and so is the timing of surgery. We as anesthesia providers can help with the use of antifibrinolytics and surgeons with meticulous surgical technique to decrease blood loss. CPB circuits are getting smaller and smaller and it is possible to reduce the prime volume with the use of techniques such as vacuum assisted drainage (VAD), venous ante grade prime (VAP), as well as retrograde arterial prime (RAP).
The second session of the morning was on Electrophysiology and Rhythm Disturbances and was moderated by Dr. Susan Staudt (Children’s Hospital of Wisconsin). The only talk of the session was ‘EP Studies: Does Anesthetic Technique Matter?’ by Dr. Johanna C. Schwarzenberger (UCLA). This was an outstanding summary of cardiac electrophysiology (EP) and anesthesiology. She spent some time talking about the common pediatric rhythm abnormalities and their frequency. She then went on to discuss some common EP procedures and finally spent some time talking about the effects of anesthetic agents on cardiac electrophysiology. She reminded us that the ideal anesthetic for EP should not alter intrinsic pacemaker function, impulse propagation, refractoriness or autonomic tone. Also it should not suppress the ability to identify aberrant pathways during attempts to trigger and simulate the reentrant arrhythmia. She concluded by stating that developing a robust understanding of the principles of cardiac electrophysiology and how the anesthesiology drugs, autonomic tone and physiologic variables affect cardiac EP will aid the anesthesiologist in choosing the appropriate anesthetic techniques for complex EP procedures.
The final session of the morning titled ‘Insights from recent literature-Three favorite papers’ was moderated by Dr. Nina Guzzetta (Emory University). The first paper was ‘Prevention of pediatric low cardiac output syndrome: Results from the European survey EuLoCOS-Paed’ Winnie Vogt et.al Pediatric Anesthesia 21(2011).The survey covered clinicians in 47 European countries and not unexpectedly there were 24 different drug regimens, with milrinone alone (23%) being the most common. The summary by the authors was that preventative drug treatment for LCOS in children following open heart surgery is highly variable and not backed by the necessary evidence for efficacy and safety.
The second favorite paper was presented by Dr. Victor C. Baum (University of Virginia Medical Center) ‘Tight Glycemic Control versus standard care after pediatric cardiac surgery’ Gaies et.al New England Journal of Medicine 2012;367. The tight glycemic control involved an insulin dosing algorithm targeting blood glucose 80-110mg/dl. The primary outcome measure was healthcare associated infections in ICU and the secondary outcome measures were mortality, cardiac ICU length of stay (LOS), organ failure and hypoglycemia. Conclusions from the paper were that tight glucose control did not change the rate of hospital associated infections, mortality or cardiac ICU LOS.
The third favorite paper was presented by Dr. Ken Brady (Texas Children’s Hospital) with an unusual title ’A randomized trial of ICP monitoring: How the literature can harm patients’. The paper in question was ‘A Trial of Intracranial-Pressure Monitoring in Traumatic Brain Injury’ Chestnut et.al. New England Journal of Medicine December 2012. Researchers in the United States, teamed with researchers in Argentina, enrolled and randomized 324 patients in Bolivian and Ecuadorian hospitals for severe traumatic brain injury to receive or not to receive intracranial pressure monitoring. The primary outcome was a composite of survival time, impaired consciousness, and functional status at 3 months and 6 months and neuropsychological status at 6 months. There was no significant between-group difference in the primary outcome. Dr Brady’s concerns related to how often have monitors been shown to improve outcome (never) yet we all know how valuable they are i.e. pulse oximetry, ECG, CVP. He concludes with the statement that monitors cannot be studied as an intervention or else we would have to throw away pulse oximeters.
Thursday, March 14 - CCAS Afternoon Sessions
By Warwick A. Ames, MBBS
Duke University
The afternoon session began with the three ‘best posters’ from those submitted and accepted to the 2013 CCAS. Moderator was Dr. Mark Twite (Children’s Hospital Colorado).
Dr. Kelly Machovec (Duke University) presented data on the use of the Heparin management system in neonates and infants, concluding that its use was associated with reduced costs and exposure to blood products. Dr. Nina Deutsch (Childrens National Medical Center) has assessed the evidence for spinal cord injury during surgical repair of coarctation of the aorta. She determined that SSEP monitoring and serum lactate can be used as indicators of low flow states and that new onset clonus is suggestive of possible motor neurone injury. Finally Dr. Nina Guzzetta (Emory University) presented data investigating the in vivo augmentation of thrombin generation in neonatal plasma following cardiopulmonary bypass, using different strategies in replacement of coagulation factors.
The following session, moderated by Dr. G.D Williams (Stanford University), was an interactive session on ‘cardiac care and patient safety’. It began with Dr. David Vener (Texas Children’s Hospital) presenting the first report from the CCAS-STS database. Having stressed the importance of teamwork, Dr. Vener detailed the ‘Anesthesia Outcomes’ from 2010-2012. Although only 25% of congenital heart programs in the United States are currently submitting ‘anesthesia’ data, the case volume was very impressive. With an incidence of ‘anesthesia-related adverse events’ at around 2%, the most common included airway mishaps (difficult intubation/re-intubation/stridor/airway compromise following TEE insertion), issues with vascular access and allergic reactions (including protamine). There are changes to the database, effective as of July 2013, which will provide better definition and include additional outcome options. Other centers currently not providing data are encouraged to join and contribute to this valuable resource.
The second lecture in the session was a joint presentation by Dr. Calvin Kuan (Stanford University) and Dr. Steve Tosone (Emory University). Focusing again on patient safety and teamwork, there was a strong emphasis on crisis resource management: the importance of communication, leadership and decision-making; the negative impact of biases, cognitive errors and hazardous attitudes. Dr. Kuan presented a dramatic fictional video scenario detailing the handoff of an unstable patient to recovery staff, in which the potential pitfalls of poor teamwork were highlighted. An alternative ending assuaged the concerns of almost all the assembled audience.
The last formal session of the day was a review of Double Outlet Right Ventricle (DORV), from cardiology, surgery and anesthesia perspectives. Dr. Komal Kamra, a cardiologist from Stanford University, gave a detailed description of the clinical spectrum of the anomaly and demonstrated this with echocardiography findings. Defined as when both great vessels arise entirely or predominantly from the right ventricle, she further elaborated on the factors that influenced the clinical presentation of the defect: such as presence and location of the ventricular septal defect (VSD), associated pulmonary or systemic outflow obstruction, the degree of pulmonary vascular resistance and other cardiac anomalies. To this end, DORV may present physiologically as either a VSD, a tetralogy of fallot or transposition of the great arteries (TGA). Dr. Kamra suggested the importance of TEE in the initial diagnosis, as a guide to repair and evaluation of surgical result.
The surgical perspective was provided by Dr. Victor Morell (Children’s Hospital of Pittsburgh), who began by stating that DORV is not the ‘usual’ VSD, impart because the relationship between the aorta and left ventricle is not normal. A typical patch repair (Rastelli) will likely result in left ventricular outflow obstruction and so depending on the physiological type, surgical repair is more complex. Factors such as distance from the tricuspid to pulmonary valve and type of VSD (uncommitted or remote) dictate the surgical options. He further eloquently described said options, such as the Kawashima operation, the ‘Bove’ approach, and the Nikaidoh procedure. Reminding the audience that the Fontan is a procedure of choice for some DORV, he concluded that any surgical repair is associated with a significant need for reoperation and can be associated with late deaths.
Finally Dr. James Spaeth (Cincinnati Children’s Hospital Medical Center) gave a review of the anesthetic management of a patient with Taussig-Bing variant of DORV (TGA and subpulmonary VSD) with aortic arch obstruction. In choosing such as a patient he demonstrated how challenging the management can be. Emphasizing the need for a thorough preoperative workup, he reviewed the physiology of the lesion, stated anesthesia goals and described his anesthetic methodology. Common clinical issues after separation from cardiopulmonary bypass include low cardiac output syndrome, rhythm disturbances, pulmonary hypertension, bleeding and poor pulmonary function were also discussed in detail.
The day concluded with a viewing and discussion of the 18 accepted posters, moderated by Dr. Robert Friesen, Dr. Jeremy Geiduschek and Dr. Robert Seal.
Thursday, March 14 - SIG - Pediatric Pain Medicine - Morning Sessions
By Karen Dean, MD
Children’s Hospital Colorado
University of Colorado School of Medicine
Acute Pain Session 1: Innovations in Regional Anesthesia
The first session on Thursday, moderated by Rosalie Tassone, MD, MPH (University of Illinois Medical Center at Chicago), was delivered by Charles Berde, MD, PhD (Children’s Hospital, Boston). Berde, perhaps best known for his research in neuropathic pain in children, led the audience on a lively quest for the “holy grail” of regional anesthesia - a better local anesthetic.
He began his presentation with a review of the major limitations of local anesthetics currently in use:
- their effect is too short in duration
- they lack specificity for sensory blockade, often causing undesirable motor and autonomic effects
- they have systemic toxicity, causing seizures and arrhythmias
- they have local tissue toxicity, leading to transient or permanent nerve deficits or muscle necrosis, especially with high concentrations or prolonged exposure
Dr. Berde then shifted his focus to a review of advances in local anesthetic technology during the last 40 years. Newly developed chiral anesthetics including ropivacaine and levo-bupivacaine have provided modest improvements in therapeutic index, but have not solved the duration problem. In order to extend the duration of local anesthesia, researchers have experimented with numerous additives (e.g. vasoconstrictors, ketamine), controlled-release vehicles (e.g. liposomes, microspheres), and novel drugs. Additives like epinephrine extend duration to a maximum of about 18 hours. Controlled-release vehicles are problematic because of issues with vehicle stability, storage and reconstitution, inflammatory responses, unpredictable duration, and low potency.
So where else can one look for a better local anesthetic? The sea. As Dr. Berde and his collaborators have demonstrated, it turns out that site 1 sodium channel blockers derived from marine toxins (e.g. tetrodotoxin, neosaxitoxin) are powerful local anesthetics with minimal neurotoxicity or cardiac toxicity. And when given in combination with bupivacaine or epinephrine, synergism results in profoundly increased duration of effect.
Another promising area of research is looking not at improving duration, but at targeting local anesthetics to the subset of nerve fibers that control pain and sensation. This can be done by allowing drug entry only into small fibers (C and A-delta fibers). Drugs like capsaicin can be used to open TRPV1 ion channels, which exist only in small sensory fibers, and these open channels allow entry of the investigational local anesthetic QX-314. Alternately, specific sodium channel subtypes may be important targets for better sensory-selective local anesthetics.
The next lecture was an update on the use of adjuvants in regional anesthesia, delivered by Corrie Anderson, MD (Seattle Children’s Hospital). Adjuvants have many potential benefits, including the ability to potentiate analgesia, alter tissue permeability and quicken onset, increase duration of effect, lower toxicity, alter block quality and density, and prevent tachyphylaxis. But it seems that for every useful adjuvant, there is also one that falls out of favor, either because of inutility or toxicity. So who can keep up? Thanks to Anderson’s thoughtful review, we all can.
The lecture began with a list of local anesthetic adjuvants, including opioids, epinephrine, clonidine, ketamine, magnesium, dexamethasone, adenosine, neostigmine, dextran, neuromuscular blocking drugs, bicarbonate, and dexmedetomidine. Then Anderson chose to focus his comments on the most important classes of adjuvants, explaining their mode of action, benefits, and toxicities.
Opioids are the most commonly used adjuvants in neuraxial anesthesia. They competitively bind to receptors and lead to hyperpolarization of afferent sensory neurons, thus inhibiting transmission of afferent pain signals. However, they also have important side effects and toxicities, including respiratory depression, nausea, vomiting, and urinary retention. Morphine, because of its hydrophilic properties, spreads cephalad in the spinal column and causes both immediate and delayed respiratory depression. Fentanyl, on the other hand, is highly lipophilic, so it easily diffuses away from the spinal cord and into the bloodstream. A continuous neuraxial fentanyl infusion is then just a really expensive mode of systemic administration.
Alpha-1 adrenergic agents like epinephrine are valued adjuvants because of their vasoconstrictive properties. By causing local vasoconstriction, epinephrine prevents diffusion of local anesthetic away from the site of action and limits systemic blood levels, thereby prolonging duration of effect and limiting systemic toxicity. However, Anderson draws our attention to a recent case series by Meyer et al. (Anesthesia & Analgesia 2012) in which prolonged epidural epinephrine exposure was implicated in at least one case of severe neurological damage. He suggests that until we have more information, it may be prudent to limit exposure of the spinal cord to epinephrine. But concerns over rare neuraxial toxicity from epinephrine must be weighed against its utility in detecting the more common occurrence of inadvertent intravascular injection during neuraxial anesthesia.
Alpha-2 adrenergic agents have been used both centrally and peripherally as local anesthetic adjuvants. In the central nervous system, they bind presynaptic receptors and decrease neurotransmitter release and sympathetic outflow. Their analgesic role in the peripheral nervous system is unclear, but is thought to stem from hyperpolarization of activity-dependent channels on sensory neurons and diminished nerve conduction. Results with clonidine have been mixed. Its addition to local anesthetic for axillary block showed little benefit in one study, while in another study clonidine was noted to significantly prolong the duration of caudal blockade (Goodarzi et al., Anesthesiology 2000). Potential side effects of clonidine have also been reported, including apnea, desaturation, and sedation.
In summary, no adjuvant is perfect – they all have potential side effects and toxicities. In the absence of overwhelming evidence to support their utility and safety in the pediatric population, adjuvants should be chosen with caution and with attention to the risks and benefits for each child.
Acute Pain Session 2: Personalizing Pediatric Pain Medicine
The concept of personalized medicine is relatively new, but as genetic science has leapt ahead in recent years, so has our understanding of how an individual’s genome might affect their response to medical therapy. The third speaker of the morning, Jeffrey Galinkin, MD, FAAP (Children’s Hospital Colorado), used his time to deftly dispel some of the mystery surround the interplay between genetics and perioperative anesthetic practice.
He began by explaining several key terms, including pharmacogenomics, epigenetics, transcriptomics, proteomics, metabolomics, and lipidomics. Pharmacogenomics is the heart of the science, and seeks to explain how variations in an individual’s genetic code lead to differences in drug transport, site of action, metabolism, absorption, and sensitivity. Even small variations or polymorphisms in the genetic code can lead to large differences in drug response, and may be a significant cause of adverse drug reactions.
A well-known example of pharmacogenetics in action is codeine. Codeine is pro-drug, requiring conversion to morphine by the liver enzyme CYP2D6 in order to exert analgesic effect. At least 10% of people are codeine non-responders due to a single nucleotide polymorphism that results in poor metabolism of codeine to morphine. Even more alarming, though, is the small population of people who are ultra-metabolizers of codeine, and are at risk for respiratory depression and death from even a single dose of drug.
Proteomics is the study of proteins and their structures, functions, expression, and interactions. It seeks not to study individual proteins, but rather the complex interactions of multiple proteins in vivo, or so-called “systems biology”. The approximately 30,000 discrete genes in the human genome encode for millions of proteins, all of which can be differentially expressed at the cellular or tissue level. Understanding how and why these proteins are expressed at certain times may shed light on disease states such as chronic pain. This young and exciting new field of science is only limited by our capacity for data interpretation.
While on the topic of personalizing pediatric pain medicine, Nathalia Jimenez, MD, MPH (Seattle Children’s Hospital) asked the audience whether we should separate our patients into racial and ethnic groups when forming an analgesic plan. The answer, perhaps, is yes. As Jimenez described current literature on the topic, she put forth a convincing argument that race and ethnicity correlate closely with health outcomes, both because ancestry is a marker for genetic variation, and because of poorly understood psychological and social factors.
Jimenez supported her argument with a review of several papers that describe the variation of pain symptoms and pain management with race and ethnicity. One paper reports that race and ethnicity affect the prevalence of pain complaints, as well as the type of pain reported, although surely there are environmental and occupational confounders. Another study shows that limited English proficiency increases reports of pain and anxiety, an effect that is reduced by the use of interpreters. In another study, Hispanic patients received less opioid than non-Hispanic patients.
In a recent study done at Seattle Children’s, 70 patients received an anesthetic according to protocol. Both Hispanic and non-Hispanic patients had similar pain scores and rescue analgesia needs, and similar morphine plasma levels. The Hispanic group, though, had three times the number of opioid side effects, a finding which could not be explained by pharmacogenetic analysis.
Jimenez reminded the audience that pain is a phenotype, and a complex one at that. Part of an individual’s pain phenotype is determined by genetic factors, and multiple candidate gene association studies are underway. Another part of the pain phenotype, however, may be determined by poorly understood socioeconomic and cultural factors, and thus may be more difficult to study.
Pro/Con Debate: Are we missing the rave? Using ketamine routinely in the operating room
Any fan of Letterman’s “The Late Show” can appreciate a good Top 10 list. And it turns out that the Top 10 list loses none of its luster when geeked out with anesthesia terminology. The debate between Constance Monitto, MD (Johns Hopkins Children’s Center, “PRO”) and Stephen Hays, MD, FAAP (Vanderbilt University Medical Center, “CON”) was lively and entertaining.
Ketamine has been around for more than fifty years, but we are still finding new uses for this “dirty” drug. Some of its well-known advantages include ease of administration, cardiovascular stability, preservation of spontaneous ventilation, and analgesic properties. Ketamine also has benefits that are less commonly known. For instance, it has anti-inflammatory properties, and may transiently decrease depression after just one dose. It has great analgesic utility in select populations, including children with chronic cancer pain, refractory postoperative pain, centrally-mediated or neuropathic pain. There is still a lot to discover about this drug.
But fans of ketamine may find their enthusiasm tempered by the “con” argument. Ketamine is a relative of a street drug, and its safety and efficacy in children is not established. It is famous for its emergence reactions and psychomimetic effects. It has not been shown to be opioid-sparing in postoperative children, as demonstrated in a meta-analysis by Dahmani et al. (Pediatric Anesthesia 2011). Also, ketamine is clearly linked to neuronal apoptosis in animal models, and there are reasons for concern over its use in children.
So what can one take away from this debate? Maybe just like a rave, ketamine isn’t for everyone. But for children with depression, chronic or neuropathic pain, or opioid-resistance, it may be just the ticket.
Thursday, March 14 - SIG - Pediatric Pain Medicine - Afternoon Sessions
By Anne Lunney, MD
Seattle Children’s Hospital
The afternoon Pain session focused on chronic pain. It proved to be lively and informative.
Pediatric Pain Medicine Expert Panel Perspectives for Treating Pain in the Child with Cancer: A Case Based Discussion - Kenneth Goldschneider, MD, FAAP (Cincinnati Children's Hospital Medical Center: Allopathic Medicine), Yuan-Chi Lin, MD, MPH (Children’s Hospital, Boston: Complimentary Medicine), and Tonya Palermo, PhD (Seattle Children’s Hospital: Pain Psychology).
The panel provided an interactive session, with discussion of the approach to the oncology patient in the chronic pain setting. The patient, a teenager had metastatic disease, which was remittent to opioids.
In the cancer pain patient opioids are accepted for disease related nocioceptive pain. When central sensitization occurs, opioids may not effectively treat pain. Dr Goldschneider addressed the importance of considering medications to address central sensitization, such as gabapentin and pregabalin, SSRI’s and SNRI’s. In this patient gabapentin and methadone were used. Methadone has a long half-life, augments serotonin and norepinephrine, and inhibits NMDA-r.
Dr Lin spoke to the importance of Acupuncture as an adjunct for treatment of pain, nausea, and anxiety. Acupuncture and acupressure does not have the side-effect profiles seen with medications. He illustrated pericardium 6, the point to decrease nausea and vomiting which every anesthesiologist should know. Steady pressure is placed three of the patients finger-breath’s, between the two flexor tendons.
He provided insight into the support found in family and community. Spiritual beliefs, community, and prayer are particularly important to many patients and their families.
Dr Palermo spoke to the patient and family, she reminded us that there is no roadmap for dealing with debilitating pain and terminal illness. Assessing where the patient and family are in their understanding of the disease as well as what their hopes and desires is important. Providing tools for managing stress, communicating with the hospital staff and medical teams, is empowering.
Neuropathic Pain: A review - Giovanni Cucchiaro, M.D. (Children’s Hospital Los Angeles)
Neuropathic pain, defined by International Association for the Study of Pain (IASP) as Pain arising as a direct consequence of a lesion or disease affecting the somatosensory system, has painful and non-painful characteristics. Non-painful characteristic such as parasthesia may coexist with painful areas. Up-regulation of genes results in spontaneous activity of A-delta and C fibers, producing allodynia, hyperalgesia, and summation progression of pain with repetitive noxious stimuli.
Peripheral sensitization, central sensitization, and hyperalgesia mechanisms were reviewed. Peripheral mechanisms included changes in the density of cutaneous innervation, increased TRPV1expression, and abnormalities in sodium channels. Central sensitization includes pathological activity of C-fibers, calcium channel changes (release of glutamate) and increased substance-P (sensitizes dorsal horn neurons), abnormal expression of the 1.3 sodium channel in the dorsal horn, and sensitization of the thalamus and primary somatosensory cortex. Allodynia is produced when non-painful A-fiber mechanoreceptors gain access to the nocioceptive system in the presence of central sensitization.
IASP treatment guidelines include tricyclic antidepressants and duloxetine (1st), gabapentin and pregabalin (2nd), lidocine patches (3rd), tramadol and opiates (4th).
Opioid Induced Hyperalgesia - Navil Sethna, MB ChB, FAAP (Children’s Hospital, Boston)
Opioid Induced Hyperalgesia (OIH), defined as a syndrome of increased sensitivity to noxious stimuli, which occurs after acute and chronic administration of opioids. The goals as outlined were to describe the underlining mechanisms of OIH, define why and when OIH occurs, and to outline the clinical evidence and relevance for pain management. As stated in his conclusion, OIH probably occurs in the clinical setting and the risk increases with higher doses of opioids.
Neuroplastic changes occur peripherally and centrally which result in sensitization of the pronociceptive pathways resulting in an increase in pain despite increased opiod dose. This phenomenon occurs regardless of chronicity of use and opioid dose.
Tolerance was described as antinocioceptive desensitization which is on a continuum with the pronociceptive activation seen in OIH. With opioid tolerance opioid is still effective at higher doses, where with OIH the pain is diffuse, changes in quality and opioids are ineffective.
Proposed mechanisms are complex and include changes in pain processing mediated by TRPV1 and cytokines peripherally, and at opioid–r, NMDA-r, NK1-r (substance P), and dorsal pain fibers centrally.
Treatment includes de-escalation of opioids, non-opioid therapies such as NMDA and alpha adrenergic antagonists, NSAIDS, and gabapentin and pregabalin, and central and peripheral nerve blocks. Pre-operatively, the addition of ketamine, magnesium, memantine, free radical scavengers (vitamin C, mannitol), systemic local anesthetics, N2O, drugs active against glial cells (minoxidil, propentofylline), and omega 3 fatty acids show antihyperalgesic affects.
Telemedicine in Pediatric Pain Medicine
Anjana Kundu, MBBS, MD (Seattle Children’s Hospital)
Dr. Kundu appropriately delivered her talk on Telemedicine using the technology in which she is and expert, and participated in the discussion session via Skype™.
This innovative form of practice is defined as the practice of medicine using electronic communication, information technology or other means between a physician in one location and a patient in another location with or without an intervening healthcare provider. Telemedicine allows for the effective and timely treatment of pain patients.
Approximately 35% of patients report chronic pain, with an estimated financial impact 600 billion dollars. Chronic pain patients have a high use of healthcare resources, and most receive their care from primary care and emergency department physicians. Greater than 50% of community-based physicians feel poorly prepared to manage pain patients.
Chronic pain has broad sweeping impact on the patient and their families including: increased anxiety, depression, missed school and poor functioning in school, sleep difficulties, and low quality of life. There are also psychosocial and financial burdens for the family.
Dr. Kundu reviewed several studies working through the University of Washington with the Native American population in Washington State and Alaska. The sessions provide expert patient care, rural provider support, case based discussion, provider educational didactics and training modules. Over a nine-year period, 3,314 persons participated in the interdisciplinary conference, with an average of 36 persons per session.
Telemedicine directly increased rural access to expert pain management, and provides a tool for case based learning and didactic education. Indirectly, the number of competent pain-care providers also increased.
Using Internet to Enhance Access to Evidence-Based Medicine Pain Interventions.
Tonya Palermo, PhD
Dr. Palermo identified the overwhelming presence (116 million Americans) and cost of chronic pain and the impact pain has on every aspect of a child’s life. Research supports psychological interventions such as relaxation, biofeedback, and cognitive behavioral therapy as being effective in reducing pain. Unfortunately, multidisciplinary pain clinics provide focused therapies and are often geographically or financially prohibitive.
In light of the PEW Internet and American Life Project report noting that 90% of adults and teens are regular internet users, implementation of on-line counseling, video-conferencing, and interactive health technologies can increase access to care.
The preliminary stages of intervention development evaluated: needs assessment, the use of Evidence Based Medicine, Beta and usability testing, and efficacy. The tool developed is the Web-based Management of Adolescent Pain (Web-Map). Web-Map uses a travel based theme to traverse the chronic pain education and coping strategies taught in the individual units. There are both teen and parent modules covering the topics of: chronic pain, behavioral skills such as deep breathing and relaxation, cognitive skills, sleep and physical activity interventions. Examples of specific parent strategies are communication and changing behavioral responses.
The program takes about one hour per week, and takes from 8-10 weeks. A PhD fellow provides feedback after each assignment. With a patient sample of 48, it was determined that behavioral interventions for pediatric pain are ideally suited for internet delivered treatment, and there is a clinically significant decrease in pain intensity and activity limitations.
Friday, March 15 - Session I
By Elizabeth S. Yun, MD
University of Wisconsin School of Medicine
Madison, WI
The Society of Pediatric Anesthesia Pediatric Anesthesiology2013 Meeting opened with the session titled Anesthetic Challenges in Childhood Obesity, moderated by Dr. Franklyn Cladis (Children’s Hospital of Pittsburgh).
Dr. Paul J. Samuels (Cincinnati Children’s Hospital Medical Center) presented the first lecture, Anesthetic Challenges in Pediatric Obesity. In this lecture he discussed the epidemiology of pediatric obesity and reviewed the associated co-morbidities and anesthesia implications. Obesity is defined by calculating the body mass index (BMI) as weight in kilograms divided by height in centimeters, although a person’s health risk is increased with a large waist girth. For adults a BMI greater 30 is considered obese. For children, BMI is more complicated since BMI changes with age. To account for the changes, gender specific growth curves are used to calculate pediatric BMI. Therefore, BMI for age greater than 95 percentile is considered obese. In America, 12 million children between the ages of 2-19 years are obese and 5% of this population is severely obese (BMI > 99 percentile). The incidence of pediatric obesity has increased but has leveled off in recent years.
The most alarming consequence of pediatric obesity is that it leads to adult obesity and its associated co-morbidities of cardiovascular issues, type 2 diabetes and shortened life expectancy. Obese pediatric patients also experience many orthopedic problems such as a higher incidence of forearm fractures, Blounts disease and SCFE issues. Other issues include obstructive sleep apnea, hypertension, depression, hepatic disease, PCOD.
Dr. Samuels then discussed anesthetic considerations for managing obese patients in the operating room. He noted these patients were at increased risk of critical airway events, longer PACU stays and unplanned hospital admissions. He also observed that while patients had difficult mask airway and airway obstruction, it was not difficult to visualize their vocal cords during intubation. In terms of fasting guidelines and the risk of pulmonary aspirations, a recent study showed no support for more rigid guidelines in obese pediatric patients.
He then discussed the impact of anesthetic agents in the obese pediatric patient. He first defined total body weight (weight on a scale), ideal body weight (an actuarial measurement) and lean body weight (weight of muscle, bone and organs). In terms of inhaled agents, recent studies suggested that emergence and recovery from inhaled agents was slower in obese patients. However these studies did not show if these patients had delayed discharge from PACU. He reviewed the available literature on the dosing effects of different types of IV anesthetic drugs on the obese pediatric patient. He summarized these findings by stating that the doses of propol for induction, nondepolarizing muscle relaxants and narcotics should be based on lean body weight. Propofol infusion and succinylcholine should use total body weight for dosing calculations. He concluded by stating that pediatric obesity is a public health problem with more of these patients presenting for all types of surgery.
The next speaker, Dr. Deborah Schwengel (Johns Hopkins Hospital), presented OSAS and Obesity: Diagnosis, Treatment and Perioperative Care. In her lecture she discussed the interaction of obesity and obstructive sleep apnea syndrome (OSAS) to cardiovascular disease and metabolic syndrome and the implications of obesity and OSAS for perioperative care. About 1-6% of all children have OSAS. The classic features of pediatric OSAS included a peak at age 2-4 years, no obesity, enlarged tonsils and less daytime sleepiness compared to adults. According to current estimates, 13-59% of pediatric OSAS patients are either overweight or obese. While lymphoid hypertrophy, craniofacial and neuromuscular issues impact OSAS, obesity has become the most prominent disease mechanism for OSAS.
In obesity related OSAS mechanical narrowing of the airway is a central problem. Mechanical narrowing occurs from fat pads, increased critical airway closing pressure, and altered chest call mechanics. Lymphoid hypertrophy may not play a large role in obesity OSAS since removing the tonsil did not improve the OSAS. Some studies suggest lymphoid proliferation due to inflammation from sleep deprivation may lead to adenoidal enlargement. In terms of neuromuscular control and obesity OSAS, these patient may have CNS mechanisms that lead to a propensity toward airway collapse and abnormal ventilator control, especially in those with severe OSAS. These issues may increase susceptibility to medication induced airway collapse and ventilation dysregulation.
She then described emerging literature on the relationship of inflammation, obesity and OSAS. There appears to be a malignant feedback loop where OSAS and obesity lead to sleep fragmentation that leads to neurohormonal changes indicating inflammation that perpetuates more obesity and OSAS. Another important consideration is that obese children may have persistent OSAS event after tonsillectomy surgery. One study suggests that 75% of obese children having surgery will have residual OSAS. Both obesity and OSAS lead to the chronic inflammation and as a result these patients are at high risk of developing atherogenesis and cardiovascular disease. The adult literature shows other long term consequences including diabetes, hypertension, daytime sleepiness pulmonary disease and DVT. One very important consequence is metabolic syndrome, most likely due to metabolically active visceral fat. This syndrome may lead to excess daytime sleepiness and sleep apnea, thus these symptoms may be a clue to more significant disease. The prevalence of metabolic syndrome in sleep disordered breathing appears to 4-10% in adolescents. There again appears to be a circular loop of obesity leading to sleep issues that then lead to inflammation that lead to metabolic problems that then worsen the obesity.
Dr. Schwengel then reviewed the issues involved in perioperative care of these patients. Preoperatively it is important to screen these patients for OSAS and metabolic syndrome by measuring waist size, glucose, HDL and triglycerides. Intraoperatively, one should be prepared to deal with airway obstruction and careful opiod dosing. Most importantly these patients have a higher incidence of lethal respiratory events after tonsillectomy and the presence of medical comorbidity was an independent risk factor for respiratory complications. Therefore their postoperative disposition needs to be discussed and planned carefully before surgery.
In summary, obesity related OSAS is a complicated problem. Obesity and OSAS are pro inflammatory states and there is a bidirectional and pernicious association between sleep apnea and insulin resistance in obese patients
The final speaker, Dr. Carroll M. Harmon (Surgical Director, Children’s Center for Weight Management at Children’s of Alabama) presented Medical and Surgical Treatment of Adolescent Obesity. He noted that CDC requirements for patients and parents including changes in diet, behavioral modification and more physical activity did not significantly decrease adolescent obesity rates, especially in the short term. He described various drugs that are available. Orlistat, a gastric and pancreatic lipase inhibitor that prevent fat absorption in the GI tract had some positive effects at decreasing BMI but these effects diminished over time. Metformin, while useful for treating type 2 diabetes, did not reduce weight. A new drug, Exenatide, a glucagon like peptide that directly targeted the brain to decrease appetite, showed promise for some weight reduction.
He then described weight loss surgical options for adolescents: Roux en Y gastric bypass (RYGB), adjustable gastric banding (AGB) and sleeve gastrectomy (SG). Patient selection criteria included a BMI greater than 35 and other comorbidities such as Type 2 diabetes, moderate to severe OSA, severe/progressive steatohepatitis and pseudotumor cerebri. Patients also needed to actively participate in an adolescent weight loss program, have family support and decisional capacity to provide informed consent for surgery. These surgeries should take place within a multidisciplinary program with specialists dedicated to treating adolescents. Other surgical considerations included, having the proper OR table for the patient, patient mobility and the use of CPAP in the PACU after the surgery.
The Roux en Y gastric bypass has been used in adolescents since the 1980’s and involves the creation of a small gastric pouch that is malabsorptive for calories, vitamins and minerals. In adolescents, short term results were a 30 % decrease in BMI, although if the starting BMI was high, the patient would still be considered obese. The results also showed improved metabolic parameters, OSA, and psychosocial functioning. Concerns with the surgery include life long diet supplementation, weight regain and postoperative complications like strictures, leak and gastric ulcers.
The next surgery is the AGB. This short operation involves placement of a ring with an inflatable balloon around the top of the stomach. While done safely in adolescents, it is not FDA approved in patients less than 18 years. The results of this surgery also showed significant weight loss, and improved health and metabolic state. However in adults there have been some discouraging results including a high incidence esophageal dysmotility, failure rate at 10 years, and a reoperation rate for band removal.
The next surgery is the SG whereby stomach size is radically reduced and food goes from the esophagus to the duodenum. This surgery can also be used as the first stage for an eventual RYGBP. There are encouraging results in the pediatric population with few complications although there is no long term data yet. A recent study with data from 109 hospitals compared the morbidity and effectiveness of all three surgeries. It appears that from a weight loss and complication rate, the RYGBP was better than SG and SG was better than AGB. A five center adolescent bariatric research consortium created the study, Teen LABS (teen longitudinal assessment of bariatric surgery) to investigate the long term effects of bariatric surgery on adolescents. More information can be found at www.cchmc.org/teen-labs
Friday, March 15 - Sessions II and III
By Zulfiqar Ahmed, MD, FAAP
Director of Pediatric Anesthesia
Anesthesia Associates of Ann Arbor
Ann Arbor, MI
Session II
The second session of the day was moderated by SPA president, Nancy Glass MD, MBA, FAAP (Texas Children's Hospital). The topic of the session was "Ethical Dilemmas in Pediatric Anesthesiology" - An Interactive Discussion. The expert moderators of the session were John Lantos, MD (Director of Pediatric Bioethics, Children’s Mercy Hospitals & Clinics Kansas City) and Robert D. Truog, MD, MA, FCCM (Children’s Hospital, Boston)
The moderators asked some pertinent ethical case scenarios, which any pediatric anesthesiologist may face. Answers were sought from the participants using the audience response system. This resulted in an active and spirited discussion. The following cases were presented with various ethical dilemmas.
Case 1: Sixteen year old undergoing thoracotomy with fentanyl infusion which was misprogrammed with sufentanil in line. As a result, patient received a significant overdose of sufentanil requiring prolonged ventilation and extended recovery room stay.
The questions posed were: would you disclose this incident to the parents? Would you choose the word mistake or error in your description? Would you apologize for this incident?
Case 2: Sixteen year old who presents for open cholecystectomy and epidural placement. The patient and mother had received information about epidural from the surgeon's office. On the day of the surgery the antagonizing father (who was not present at the surgeon's office visit) refuses to consent for epidural and begins to threaten legal implications if an epidural is placed. The questions raised by the experts were: Would you proceed and place the epidural any way as the patient and mother have agreed and consented for the epidural or would you not perform epidural? Other options included calling security.
The rights of a sixteen year old were discussed as well as the role of surgeons in conflict resolution and medico-legal consequences in case a complication occurs.
Case 3: Sixteen year old for a thoracotomy with significant blood loss. Resuscitation efforts with blood and fluid seem to be ineffective. Significant cardiopulmonary compromise was recorded and CPR started until a disconnected femoral line was diagnosed. Reconnection lead to stable hemodynamics and the procedure was successfully finished.
The questions posed to the audience were: would you disclose such an incident to the parents especially when the patient needed CPR due to line disconnection? Would you consider the use of words error vs. mistake? Would you apologize for such incident?
Case 4: Four year-old presented for a procedure requiring a general anesthetic. The questions raised by the experts were: Do you discuss the potential of anesthetic related neurotoxicity: always, sometimes, rarely and only when asked? Would you mention the statements from AAP 2012, which mention that general anesthetics may cause learning disabilities?
Session III
The AAP Advocacy Lecture speaker was Tom Robinson, MD MPH (Solution Science Laboratory Center for Health Weight at Stanford University Medical Center).
According to Dr. Robinson, the obesity trends in U.S. population have risen dramatically over the past two decades. Every year a company like McDonald's spends 2 billion dollars on advertising fast food. Armed with powerful images, it has been shown that the same food, when presented to subjects with a McDonald's label, was reported to be better tasting (Robinson TN, et al. Arch Pediatr Adolesc Med 2007;161:792-7).
Dr. Robinson showed a long list of comorbidities associated with obesity and noted the cost of obesity to society at present is estimated to be $150 billion per year. Obesity also presents a national security issue, as about a third of the U.S. population is not medically fit to serve in the armed forces. Reduction in television watching has been the most effective intervention in waist size reduction (Robinson, TN. JAMA 1999;282:1561-7). How to influence the waist size in children and adolescent has been a focus of a number of community outreach programs where children receive "Stealth Interventions" where physical activity/reduced inactivity or diet changes are side effects of the intervention. These interventions are geared to identify target behaviors that are motivating in the target population.
Friday, March 15 - Session IV
By Jorge A. Galvez, MD
Children’s Hospital of Philadelphia
Philadelphia, PA
SPA Session IV Review - Anita Honkanen and Samuel Wald
Anita Honkanen (Lucile Packard Children’s Hospital at Stanford) and Samuel Wald (Mattel Children’s Hospital) conducted a review of the American Board of Anesthesiology’s (ABA) Maintenance of Certification in Anesthesiology® (MOCA) requirements as they pertain to pediatric anesthesiologists. An important highlight is that all individuals who will pursue the Pediatric Anesthesiology certification must complete MOCA recertification beforehand. One of the MOCA requirements involves participation in a team simulation for crisis management, also known as Anesthesia Crisis Resource Management (ACRM). There are 33 sites throughout the country that provide this service. To date, 1200 individuals have completed the simulation courses and 100% report that it was a positive learning experience.
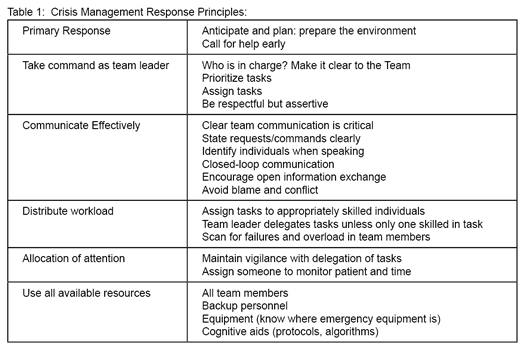
The primary goals of the ACRM process are to demonstrate appropriate team and resource management, and emphasize effective communication skills. The ACRM system is derived from the Crew Resource Management (CRM) process in the aviation industry. CRM focuses on effective crew and resource management during a simulated crisis (See table 1). Ultimately, the goals are to detect problems early in evolution and prevent adverse outcomes. In order to protect educational content, participating individuals sign a confidentiality agreement with MOCA before entering the simulation.
The typical day at a MOCA certified simulation center allows six individuals to become familiar with the simulation environment, participate in simulations under different roles, and conclude simulations with a debriefing. Each individual participates in the “hot seat” as the anesthesiologist at least one time during the day. Jonathan Anson wrote an article on the ASA Newsletter highlighting the importance of reviewing critical skills such as intraosseous lines. Following his completion of the simulation, he employed the intraosseous line during resuscitation. He wrote that his experience at MOCA empowered him to pursue the line early in the course and attributes the successful resuscitation to MOCA. Prior to that day, he had never placed an intraosseous line.
Drs. Honkanen and Wald then proceeded to the simulation portion of the session. An operating room environment was set up on the stage, including a simulation mannequin, an anesthesia machine, surgical drapes and an entire operating room team. The simulation participants included an allstar team: Anita Honkanen, Michael Chen, Rebecca Claure, RJ Mamamurthi, Ellen Wang, Jen Wagner, Nancy Glass, Kirk Lalwani and Tom Caruso.
While the simulation was being configured, the audience watched a video that set the stage for the case. A spoof of the movie “The Hangover” depicted a young man who, upon awakening in a strange bathtub in Las Vegas, realized that he unknowingly sustained an injury to his left arm. The scene fast-forwarded to an operating room where the surgeon was concluding a procedure on the patient’s left arm. The surgeon was concerned about the integrity of vascular supply to the arm and requested that the patient be evaluated in interventional radiology. The simulation involved a transfer of care to the on-call team prior to transporting the patient. There were additional anesthesiology providers available to respond during an emergency.
As one would expect in Vegas, the odds are always in favor of the house. In this case, the participants were working with a malfunctioning mannequin and monitors. The mannequin and monitors had been functioning until just a few minutes before the simulation started and were only visible to the audience on the overhead projectors. Despite the equipment problems, the team conducted the simulation very well. A great deal of time was spent providing reports of the situation to incoming staff. During the time that the team members were communicating about the history and the plan for transport, the patient began to develop hypotension and tachycardia. The anesthesia machine continued to alarm with elevated inspiratory pressures. The team aborted the transport and summoned help to the operating room.
The following events unfolded quickly. The patient’s blood pressure continued to decrease while heart rate increased. The team initiated a blood transfusion to manage the hypotension. At this time, ST depressions were noted on the ECG, which led the team to initiate chest compressions and administration of intravenous epinephrine. The patient stabilized after chest compressions and the team re-assessed the patient. The team identified absence of breath sounds on the left thorax and suspected the possibility of a left tension pneumothorax. The team performed needle decompression of the left chest with relief of air. The patient stabilized after the therapeutic maneuver and the simulation concluded.
Immediately after the simulation, a structured debriefing of the team was initiated. Multiple team members noted that they actually forgot that they were on the stage during the simulation. Once the simulation started, individuals began role-playing and truly engaged in the simulation experience.
The first audience member comment was related to the adequacy of the handoff report by the primary anesthesiologist. While all of the required information was provided in the report, it was unstructured. The audience was polled on the utilization of a formal sign-out document. The majority of participants reported that a formal document was not used at their institutions to facilitate handoffs. One participant mentioned that a formal document is used in their adult practice, but not in pediatrics. The audience response system indicated that only 17% of respondents used a formal sign-out document in their practice during transfer of care between anesthesiology providers. The presenters highlighted the availability of a handoff tool at the SPA homepage (http://www.pedsanesthesia.org/newnews/Intraoperative_Handoff_Tool_FINAL.pdf).
The team discussed the usefulness of simulating critical events such as a tension pneumothorax. The audience was polled (approximately 200 respondents) about managing tension pneumothorax: 50% respondents had managed at least one, 30% of respondents had managed two, and the remaining 20% had managed a range between zero and more than two. The audience was also polled on management of anaphylaxis, intraoperative cardiac arrest and malignant hyperthermia. Even the most experienced individuals (up to 1-13% of respondents across all questions) had only managed 5-10 cases of each scenario in their career. This highlights the importance of structured simulation environments to build on teamwork in crisis management.
The team addressed the progressive physiologic deterioration that led to the initiation of CPR. While vital signs were difficult to see for the simulation team due to malfunctioning monitors, they had noted the hypotension and tachycardia. The team commented that the emergency response was disorganized and the team was confused; there were multiple individuals attempting to direct the medical management, and the concentration of medications used was unclear. To be fair, this was an ad-hoc team without a clear structure of command that performed on stage with malfunctioning equipment.
The discussion also focused on resource management. Throughout the simulation, individuals continued to enter the room to assist in various tasks. However, when the crisis began, various team members continued to request assistance from one individual, the circulating nurse. There were times that the circulating nurse was handling several requests while other individuals in the room were not actively assisting in any tasks. During a real crisis, it is important to delegate tasks to all members of the team to ensure that the workload is balanced and the team can perform efficiently.
The purpose of the demonstration was to provide a sample of what one can expect during a MOCA simulation session. The focus of MOCA is on team dynamics and appropriate personnel and resource management during a crisis. The simulation team is challenged with a crisis scenario, and then each participant has the opportunity to be in the “hot seat”. After the scenario is completed, a structured debriefing takes place to review the areas where the team performed well and areas that could be improved.
This particular scenario highlighted the importance of handoffs as critical periods where mistakes can occur. Furthermore, there was focus on the importance of effective communication strategies as well as the ability to delegate tasks effectively to ensure that responsibilities are shared appropriately.
Friday, March 15 - Session V
By Chris Glover, MD
Texas Children’s Hospital
Houston, TX
The refresher course lectures on Friday covered the relatively broad topic of cardiomyopathies as well as management tips and tricks for patient controlled analgesia (PCA).
Dr. Nina Deutsch (Children’s National Medical Center) presented a comprehensive review on the etiology and classification of cardiomyopathy in children. Discussion also centered on the pathophysiology and anesthetic management of the various subtypes of this disorder. At its most basic form, cardiomyopathy is a disease of the myocardium which results in some form of cardiac dysfunction. Data extrapolated from the pediatric cardiomyopathy registry lists the incidence of primary pediatric cardiomyopathy as 1.13 cases per 100,000 children. This incidence is drastically higher in infants with a rate of 8.34 cases per 100,000 children [1].
Pediatric cardiomyopathies are the leading cause of heart transplants in children aged 1 to 10 and the second-leading cause in children less than one year of age and in children aged 11 to 17 [2].
The phenotypic presentation of cardiomyopathies is quite numerous and can be classified into three broad categories: primary, acquired, and multisystem disease (see table).
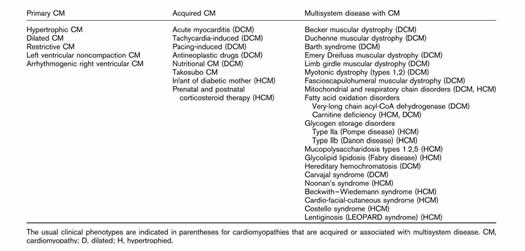
WHO classification for cardiomyopathy is listed below:
- Dilated (idiopathic, infectious, and inflammatory) - 60%
- Hypertrophic - 25%
- Ventricular noncompaction - 9%
- Arrhythmogenic - 4%
Dilated cardiomyopathy occurs most frequently and is seen in 60% of cases. Dilated cardiomyopathies are defined as an enlargement of all chambers of the heart with resultant remodeling and dilation of the ventricles leading to a more spherical LV and increased wall stress. Because of biventricular dilation, the ejection fraction and cardiac output are both decreased resulting in increases in the preload (LVEDP) and atrial filling pressures. This subsequently puts patients at risk for developing ventricular arrhythmias and congestive failure. The etiology of dilated cardiomyopathy is idiopathic in 2/3 of patients while the remaining 1/3 of patients develop dilated cardiomyopathy from myocarditis (immune mediated following viral infection), familial causes, or neuromuscular disorders [3]. Pertinent points on preoperative assessment of children with dilated cardiomyopathy include a detailed history with specific emphasis on constitutional symptoms such as generalized malaise, tachypnea, or poor feeding. Physical exam should look for the presence of jugular venous distension, hepatomegaly, or a diastolic gallop. Recent echocardiography is mandatory in these children. Lab assessment and review of medications are also warranted.
Anesthetic management should be centered on the premise that because of hypovolemia, current pharmacologic therapy, and underlying heart failure; patients are at high risk for acute deterioration with anesthestic induction [2] as the poorly contracting LV is unable to maintain the cardiac output. The use of low dose inotropes (milrinone, dobutamine, low dose epinephrine) may be required perioperatively [4]. A study on the complication profile in children with cardiomyopathy undergoing noncardiac surgery points to complications occurring in 83% of patients sampled with mortality occurring at a rate of 3% [5]. Anesthetic goals include maintaining the preload and ensuring a normal diastolic pressure to maintain coronary perfusion. Every attempt should be made to avoid tachycardia, increased afterload, or decreases in myocardial contractility. To achieve these goals, induction agents that are not myocardial depressants such as etomidate should be the preference. For longer cases, Dr. Deutsch advocated for invasive monitoring, use of inotropes, and postoperative ICU monitoring.
Hypertrophic cardiomyopathy (HOCM) is the leading cause of sudden death in children with idiopathic and familial conditions accounting for 75% of cases [4]. This condition results in increased regional or global wall thickness with the potential for the development of focal hypertrophy around the coronaries. As the musculature of the heart increases, you see a decrease in both the size of the ventricular cavity and the ability for diastolic relaxation. It is important to note that the ejection fraction can be normal despite poor function. Symptoms seen with HOCM include pulmonary congestion, myocardial ischemia, and decreased cardiac output. Tachycardia is very concerning as it can result in sudden death. The preoperative evaluation should include an echocardiogram with emphasis on LV mass. Infants with LV mass index >150g/m2 have a predilection for perioperative arrhythmias and myocardial ischemia with tachycardia. Propanolol and calcium channel blockers should be continued during the perioperative period. Diuretics and ACE – inhibitors are to be avoided as they increase the left ventricular outflow tract (LVOT) gradient.
The goals of the anesthetic are to optimize diastolic filling time via heart rate control (sinus) and minimize increases in the left ventricular outflow tract obstruction (LVOTO). Maintenance of SVR and prevention of hypovolemia are the best means to accomplish this. Volatile anesthetics and etomidate are well tolerated in most patients. Propofol with its profound effects on the preload and SVR does not seem an ideal choice. Ketamine does a decent job maintaining the diastolic pressure, but the associated tachycardia with its use also points to looking for an alternative agent in this patient population. If hypotension is encountered through the course of the anesthetic, low dose phenylephrine and beta blockade should be administered. Inotropes should be avoided as they can worsen the LVOTO and decrease the cardiac output.
Left ventricular non-compaction occurs secondary to an arrest in the maturation of the myocardium during embryogenesis and is associated with mitochondrial and neuromuscular disorders. Symptoms of LV non-compaction mimic dilated cardiomyopathy, but this condition is associated with worse outcomes. Heart failure, arrhythmias, mural thrombi, and ventricular dysynchrony can all occur in patients with this condition. The pharmacologic treatment for this disorder entails use of ACE – inhibitors, Beta –blockers, diuretics, and inotropic support with milrinone. Dr. Deutsch advised screening for neuromuscular disease as well. If ventricular dysynchrony is encountered, biventricular pacing has been shown to be of benefit [4].
Dr. Deutsch then covered arrhythmogenic right ventricular cardiomyopathy (ARVC) and restrictive cardiomyopathy. ARVC is caused by a defect in the cardiac desmosomes. The end result is that the RV is infiltrated with fibrous and fatty tissue resulting in regional or global hypokinesis. The presentation for this condition can be encountered in adolescents complaining of palpitations, syncope, or atypical chest pain. Sudden death can be seen in these patients. The course of this disorder covers four phases: concealed, overt arrhythmogenic, isolated right heart failure, biventricular failure. The therapy for this condition is placement of an ICD and radiofrequency ablation. Anesthetic considerations include the avoidance of catecholamines, placement of Zoll pads prior to induction, and continuance of anti-arrhythmic drugs.
Restrictive cardiomyopathy results from endomyocardial fibrosis leading to myocardial stiffness and impaired ventricular filling. This condition is thought to be caused by a gene mutation of the cardiac sarcomere. This progressive disorder causes decreased stroke volume and decreased cardiac output with resultant increases in the pulmonary vascular tree. Echocardiographic findings will display small ventricles with massively dilated atria. Jugular venous pressures will be elevated as is the pulmonary vascular resistance. The cardiac output is dependent on the heart rate in this condition do inotropic agents (milrinone, dobutamine) have utility. Avoidance of increases in the PVR is the primary anesthetic goal.
Dr. Deutsch concluded the talk by pointing out that there are multiple forms of cardiomyopathy. The need for a balanced anesthetic is imperative for good patient outcomes in this patient population especially given they are surviving for longer periods as medicine and technology improve.
Dr. Shobya Malviya (CS Mott Children’s Hospital) covered the later session and covered tips and tricks in the use of PCA and some of the evidence behind its use.
A brief history on the evolution of PCA pointed out its initial use in adults during the early 1970s with a gradual transition to use in pediatrics by the late 1980s. Advantages for the use of this pain control modality center on its ability to give autonomy to patients/parent’s while allowing for a tailored approach with regards to opioid use. Basal infusions lead to improved sleep and pain scores, but monitoring is imperative to minimize side effects.
Dr. Malviya covered the benefits and pitfalls with the institution of basal infusions in pediatric patients via case discussions. Benefits include maintenance of therapeutic opioid concentrations, improved sleep, improved analgesic efficacy, reduction in overall opioid consumption, and fewer side effects. The risks associated with continuous infusions center on potential for oversedation and concerns of safety with continuous use. Literature review reveals conflicting data on use of continuous infusions in children. As such, Dr. Malviya’s recommendations center on the use of continuous infusions in those with
- Cancer pain or mucositis
- Children with sickle cell disease related pain
- Major surgery (thoracic, spinal fusion)
as well as vigilance in monitoring via the use of pulse oximetry, respiratory status, and sedation depth.
An interesting slide mentioned by Dr. Malviya reported the use of ketamine as a PCA agent. Study results cited from a South Korean study on pediatric patients undergoing Nuss bar placement revealed lower pain scores, reduced fentanyl use, decreased PONV, and an absence of respiratory depression or psychosis [6]. Also discussed was the use of acetaminophen via PCA, with a noted decrease in overall opioid use approaching 50%.
Recommendations for the management of PCA included the presence of a multidisciplinary pediatric pain service and vigilance in the care of these children. Methods to achieve this include rounding twice daily, training and inservices for the nursing staff, availability of super users, and minimizing distance management over the phone.
With regards to PCA by proxy (nurse controlled versus parent controlled), Dr. Malviya cited an observational study covering 220 patients where pain relief was effectively achieved in 80% of the patients followed. However, this study pointed out a 4% use of opioid reversal with 1.8% of children experiencing apnea.[7]. Another study mentioned during the lecture noted approximately equivalent rates of adverse effects (24% PCA; 22% NCA) with these methods. Naloxone use in preschoolers and developmentally delayed children ranged between 2-3%[8]. A Joint commission alert in 2008 sent out after 15 medical errors resulting in harm/ death from NCA required formulation of specific policies and procedures for use of PCA by individuals other than patient.
In another prospective study of 10000 children, respiratory depression and sedation incidence approached 4.5% and severe adverse effects were noted in 0.4% [9]. Study data points to neonates as well as developmentally delayed children >11 years of age being at highest risk. A rather sobering point in the lecture was a quote by the Physician-Patient Alliance for Health and Safety approximating 700 deaths and 56000 adverse events over a four-year period attributed to the use of PCA pumps.
Errors were then discussed. Not only is human error and equipment failure concerning, but the concomitant use of sedatives in children with PCA. Patient conditions (obesity, OSA) should always play a role in decision making. Monitoring requirements are based on recommendations of the Anesthesia Patient Safety Foundation (APSF) although cur rently, there remains a lack of consensus with regards to monitoring in children on PCA. Continuous pulse oximetry and continuous measure of respiratory rate with reliable alerting mechanisms should be used. Assessment of sedation with use of the UMSS was discussed as well (ability to complete sentences; orientation, respiratory pattern).
In summary, Dr. Malviya reports the overall use of PCA via patient, nurse, or parent is relatively safe. Multimodal analgesia should always be used as their opioid sparing effects should decrease the potential for minor as well as severe adverse effects. The advocation of frequent assessment, use of technology, careful consideration of dosing should minimize the potential issues of respiratory depression and other opioid complications. This should continue contributing to the overall safety profile seen thus far with the use of PCA in pediatric patients.
References
- Wilkinson, J.D., et al., The pediatric cardiomyopathy registry and heart failure: key results from the first 15 years. Heart Fail Clin, 2010. 6(4): p. 401-13, vii.
- Rosenthal, D.N. and G.B. Hammer, Cardiomyopathy and heart failure in children: anesthetic implications. Paediatr Anaesth, 2011. 21(5): p. 577-84.
- Sagar, S., P.P. Liu, and L.T. Cooper, Jr., Myocarditis. Lancet, 2012. 379(9817): p. 738-47.
- Ing, R.J., W.A. Ames, and N.A. Chambers, Paediatric cardiomyopathy and anaesthesia. Br J Anaesth, 2012. 108(1): p. 4-12.
- Kipps, A.K., et al., Children with cardiomyopathy: complications after noncardiac procedures with general anesthesia. Paediatr Anaesth, 2007. 17(8): p. 775-81.
- Cha, M.H., et al., Beneficial effects of adding ketamine to intravenous patient-controlled analgesia with fentanyl after the Nuss procedure in pediatric patients. Yonsei Med J, 2012. 53(2): p. 427-32.
- Monitto, C.L., et al., The safety and efficacy of parent-/nurse-controlled analgesia in patients less than six years of age. Anesth Analg, 2000. 91(3): p. 573-9.
- Voepel-Lewis, T., et al., The prevalence of and risk factors for adverse events in children receiving patient-controlled analgesia by proxy or patient-controlled analgesia after surgery. Anesth Analg, 2008. 107(1): p. 70-5.
- Howard, R.F., et al., Nurse-controlled analgesia (NCA) following major surgery in 10,000 patients in a children's hospital. Paediatr Anaesth, 2010. 20(2): p. 126-34.
Saturday, March 16 - Session I
By J. Grant McFadyen, MBChB, FRCA
Seattle Children’s Hospital
University of Washington School of Medicine
Seattle, WA
The first session on Saturday, moderated by Mark A. Rockoff, MD (Boston Children’s Hospital), focused on advances in pediatric neuroanesthesia. First, Nicholas Carling, M.D. (Texas Children’s Hospital) discussed the challenges of providing anesthesia for intraoperative brain mapping and deep brain stimulation in children. Intraoperative brain mapping techniques, such as electrophysiological monitoring and neurocognitive testing, aid in aggressive resection of tumors or epileptic foci adjacent to areas of functional or “eloquent” cortex, while attempting to minimize neurological deficits. The demands of electrophysiological monitoring have a profound effect on the anesthetic technique and agents that can be used during the procedure. In some instances the patient must be awake, such as when language testing is being performed. The ideal anesthetic technique would keep the brain as active as when the patient is awake.
The lack of prospective, randomized trials comparing anesthetic techniques has resulted in protocols that vary by institution, and there is no consensus as to the optimal anesthetic technique for mapping procedures. This was borne out by the audience’s response to the question “A 12 year old patient with intractable epilepsy is scheduled for resection of the seizure focus with intraoperative electrocorticography and functional mapping. The most appropriate anesthetic approach is…?” 34% chose MAC with propofol/remifentanil, while 39% chose an Asleep/Awake/Asleep technique. The take home message is that the anesthetic plan needs to be individualized for each patient.
Electrocorticography (ECoG) is utilized during epilepsy surgeries to help identify abnormal EEG patterns that result from epileptogenic foci. When used to maintain general anesthesia during ECoG monitoring, low levels of volatile anesthetic combined with higher dose opioid administration should not interfere with ECoG. Propofol should be discontinued 20 minutes prior to the start of ECoG. Dexmedetomidine has minimal effect on ECoG and can be continued during recordings at low infusion rates. Because both volatile agents and propofol are discontinued or used in very low doses during brain mapping, there is potential for patient awareness, discomfort, and movement. Using large doses of short-acting opioids, one can maintain patient comfort, minimize the chance of patient movement, and not affect brain mapping. Dr Carling suggested the following agents for GA: opioid Infusion in combination with volatile agent, N2O, or propofol. Sufentanil 0.2-1 mcg/kg/hr or Remifentanil 0.05-1 mcg/kg/min. Volatile agent: low dose (<0.5 MAC). Propofol 100-250 mcg/kg/min (discontinue prior to mapping). Dexmedetomidine 0.2-0.7 mcg/kg/hr.
Direct cortical stimulation is the process of applying direct electrical stimulation to the cortex to help map eloquent areas responsible for motor function, language, vision, or sensation. Of these, only motor mapping may be performed under general anesthesia, without the use of neuromuscular blockers. The decision to perform an awake craniotomy is not an easy one. Risks and benefits need to be discussed with the surgeon, and patients need to be carefully selected. The importance of a thorough preoperative assessment and communication with the child cannot be overemphasized. The patient should have a clear understanding of what will be done, what he/she will experience, and be reassured that they will be kept comfortable and safe.
Deep brain stimulation (DBS) involves the placement of electrodes into deep nuclei of the brain, with common targets including the globus pallidus internus and subthalamic nuclei. The most common indications for DBS in the pediatric population include movement disorders such as dystonia and Tourette’s. The challenge with DBS procedures concerns balancing the desire for an awake patient with a patient population that, because of their disease process, can be difficult to manage with an awake technique.
Two common methods for “awake craniotomy” include local anesthetic with conscious sedation and the Asleep/Awake/Asleep technique, whereby general anesthesia is induced and then completely stopped during the time of mapping.
Propofol during the sleep portion, dexmedetomidine 0.5-1 mcg/kg/hr during the asleep portion and 0.1-0.5 mcg/kg/hr during the awake portion, and remifentanil are suggested. Tips for successful awake craniotomy include local anesthetic scalp block, antiemetic, an LMA, a BIS monitor and careful patient positioning and padding.
Dr. Carling showed an amusing Xtranormal cartoon clip satirizing the different expectations of two members of an OR team.
The next speaker, Charles Haberkern, MD, MPH (Seattle Children’s Hospital), discussed anesthetic management for craniosynostosis repair. He gave an interesting historical and descriptive overview of the condition, which has an incidence of 1:2000 and can occur in isolation or as a component of a syndrome. The sagittal suture is the most commonly involved, accounting for 40 to 50% of cases, followed by the coronal suture in 20 to 30%.
The pathogenesis of craniosynostosis involves failure of normal interplay between developing brain, cranial bone, and dura, and can result from genetic mutations. Apart from resulting in an altered head shape, untreated craniosynostosis may result in hydrocephaly and increased intracranial pressure (15% in single suture, 50% in syndromic patients), developmental delay, visual changes or upper airway obstruction.
Dr. Haberkern discussed the various surgical options, and pointed out that the newer “non-invasive” procedures result in less blood loss, fewer complications, decreased need for ICU care and shorter surgical duration.
He then addressed the perioperative concerns in managing these cases. Preoperatively, particular attention needs to be paid to airway assessment and blood product preparation. A thorough cardiac evaluation needs to be performed, particularly with respect to looking for a PFO/ASD. If one is present, consideration of closure prior to craniosynostosis repair needs to be made. Intraoperatively, meticulous attention should be paid to securing of the airway, adequate vascular access and monitoring, proper positioning, blood products, ICP, and to metabolic/ physiologic changes. 17% of the audience indicated that they always use precordial Doppler monitoring, 51% that they never do. 87% indicated that they always insert an arterial line. In a study by Faberowski et al, Anesthesiology 92:20, 2000, venous air embolism (VAE) occurred in 83% of cases. 48% were grade 1 (Doppler changes), 36% grade 2 (end-tidal CO2 changes) and 16% grade 3 (hypotension). A viscous cycle can ensue, with hypotension leading to VAE, which can result in worsening hypotension.
In the postoperative period ventilatory support may be needed. Analgesia needs to be provided. Anemia and coagulopathy may need to be corrected, and vigilance paid to the possibility of metabolic changes, especially hyponatremia.
Controversies surrounding the anesthetic management of craniosynostosis include transfusion practices, controlled hypotension, perioperative EPO, activated Factor VII and tranexamic acid. 27% of the audience responded that they always use antifibrinolytics and 23% that they occasionally do. 62% of respondents felt that a dedicated craniofacial team improves outcomes.
Dr. Haberkern concluded by drawing attention to the Pediatric Craniofacial Surgery Perioperative Registry (PCSPR), under the auspices of Paul Stricker, MD (Children’s Hospital of Philadelphia)
The third speaker, Tod Sloan, MD, PhD, MBA, (Children’s Hospital Colorado), gave an informative talk on intraoperative neural monitoring in pediatrics. He noted that maturation of the nervous system evolves after birth until adolescence, with the majority of changes under age three.
Somatosensory evoked potential (SSEP) monitoring has been used since the 1970s. SSEP monitors transmission in the peripheral nerve stimulated, in the posterior spinal cord, in the brainstem medial lemniscal pathway, in the thalamus, and the response of the sensory cortex. SSEP monitoring is useful for spine, brainstem and brain surgery. With regard to age, SSEP evolves with maturation until adult values are reached at age three to fouryears. Under age three, the nervous system is more affected by temperature and physiological effects. Under age three anesthesia effects are marked and ketamine may be helpful. At adolescence the responses are better than in adults.
Motor evoked potential (MEP) monitoring measures synaptic transmission in the spinal cord grey matter (corticospinal tract), transmission in a peripheral nerve, through the neuromuscular junction, and response of the muscle motor units. MEP is used for cortical vascular surgery, to “map” the motor cortex in asleep patients, to identify ischemia with vascular surgery, and to determine brainstem integrity (collision studies), spinal cord integrity (spine and intramedullary surgery) and peripheral nerve integrity. MEP is very sensitive to spinal cord ischemia. As far as age is concerned, there is concern about the use of electrodes on the head in babies under 18 months of age, because of the open fontanelles. There is a higher stimulation threshold up to three months. Facilitation methods may be needed for MEP under age three, as is supportive anesthesia. Central and peripheral myelination continues to adolescence.
Auditory brainstem responses (ABR) are used to preserve the integrity of the cochlea, c.n. VIII, for hearing preservation with surgery, and to preserve general brainstem viability. ABR is useful in infants, and adult values are reached by age three.
Electromyography (EMG) is a simple recording technology to assess nerve roots and reflex pathways, and to assess nerve injury during spontaneous activity. EMG in the very young may resemble a myopathy. In infants and newborns neuromuscular transmission may suggest a transmission defect. Peripheral nerve myelination proceeds until age three to five with near adult conduction velocity by age three.
Dr. Sloan concluded by saying that intraoperative monitoring is considered the standard of care for scoliosis correction/complex spine surgery and for acoustic neuroma resection, to facilitate facial nerve preservation. Indispensible monitoring includes electrocorticography in epilepsy surgery, cortical mapping of the sensory-motor sulcus, brainstem mapping of cranial nuclei and “safe entry zones” to deeper structures, EMG mapping in dorsal rhizotomy for spasticity and in tethered cord surgery, and for peripheral nerve procedures.
Saturday, March 16 - Session II - Poster Awards
SPA Young Investigator Awards
1st Place: Vanessa Olbrecht, MD
Novel Genetic Variants of Organic Cation Transporter 1 Explain Differential Clearance of Morphine in Children and Unequal Burden of Perioperative Pain and Opioid Adverse Effects
Olbrecht, V, Chidambaran, V, Fukuda, T, Mizuno, T, Venkatasubramanian, R, Esslinger, H, Vinks, A, Sadhasivam, S
Cincinnati Children's Hospital

Dr. Kirk Lalwani, Program Chair, presents the 1st place
Young Investigator Award to Dr. Vanessa Olbrecht.
2nd Place Khaled Dajani, MD
Prospective evaluation of pre-operative predictors of difficult intubating conditions in a pediatric population: A study of 1000 ambulatory surgery patients.
Dajani, K, Hopkins, P, Paek, J, Manyang, P, Capehart, S, LaGrone, C, Benevides, S, Servier, N, McWilliams, V, Emerald, J, Diaz, L, Stayer, S, Olutoye, O
Texas Children's Hospital, Baylor College of Medicine

Dr. Kirk Lalwani, Program Chair, presents the 2nd place
Young Investigator Award to Dr. Khaled Dajani.
AAP Resident Research Awards
1st Place: Rajeev Subramanyam, MD
Anesthesia and Opioids related Malpractice Claims following Tonsillectomy in USA: LexisNexis Claims Database 1984-2012
Subramanyam, R, Chidambaran, V, Ding, L, Sadhasivam, S
Cincinnati Children's Hospital

Dr. Joseph Tobias, AAP Section on Anesthesiology and Pain Medicine Chairperson-Elect, presents the 1st place Resident Research Award to Dr. Rajeev Subramanyam.
2nd Place: Kim Strupp, MD
Malignant Hyperthermia Data Sniffer
Strupp, K, Wilder, R, Flick, R, Kor, D
Mayo Clinic

Dr. Joseph Tobias, AAP Section on Anesthesiology and Pain Medicine Chairperson-Elect, presents the 2nd place Resident Research Award to Dr. Kim Strupp.
3rd Place: Brent McNew, MD
Risks of Noncardiac Surgery and Other Procedures In Children with Complex Congenital Heart Disease
Watkins, S, Donahue, B, McNew, B
Vanderbilt University

Dr. Joseph Tobias, AAP Section on Anesthesiology and Pain Medicine Chairperson-Elect, presents the 3rd place Resident Research Award to Dr. Brent McNew.
Saturday, March 16 - Session III
By Erin S. Williams, MD
Texas Children’s Hospital
Baylor College of Medicine
Houston, TX
The delicious flavors and aromas of the SPA lunch permeated senses while Session III attendees enjoyed fast paced, high yield presentations regarding interesting and important topics in pediatric anesthesia. This session, “Ask the Experts Panel and Lunch,” allowed SPA attendees to enjoy a wonderful lunch and a fantastic learning experience.
Lynne Gerson Maxwell MD, FAAP (Children’s Hospital of Philadelphia), moderated the session. The three speakers included Nancy S. Hagerman M.D. (Cincinnati Children’s Hospital Medical Center), Christie D. Burnett-Yarnell, M.D. (Arkansas Children’s Hospital) and Priscilla J. Garcia, M.D. (Texas Children’s Hospital). Each of the talks included fifteen minutes of power-packed review of the management of patients with pulmonary, hepatic, or renal diseases.
The first speaker, Dr. Hagerman provided a phenomenal lecture regarding the perioperative implications of pulmonary disease. During her talk she discussed the anatomical and structural abnormalities of the pulmonary system, chronic lung diseases, and acute infections. She also gave the audience insight as to the current treatment of patients with obstructive sleep apnea (OSA), bronchomalacia, asthma, cystic fibrosis (CF) and upper respiratory tract infections.
The second presenter, Dr. Burnett-Yarnell gave an insightful talk, rich with information. Her talk reviewed the very complex topic Anesthetic Implications of Liver Disease in Children. This lecture reviewed the anesthetic management of patients with cholestasis, impaired development such as biliary atresia, Alagille syndrome, non-alcoholic fatty liver disease, and coagulopathies. Dr. Burnett-Yarnell also reminded the audience of the overall systemic effects of liver disease and gave helpful management tips.
The final lecturer, Dr. Garcia provided an excellent review of the very intricate topic Anesthetic Implications of Renal Disease. Her outstanding talk highlighted the pre-operative, intra-operative and post-operative phases of anesthetic management of such patients. Dr. Garcia’s talk also addressed the important aspects of the history and physical exam as well as provided guidance regarding labs and intravenous fluid management. Finally, the new audience response program was incorporated into the presentation; this useful tool definitely encouraged audience participation.
Overall this session was outstanding! The Ask the Experts Panel and Lunch was an excellent learning experience for all who attended.
Saturday, March 16 - Session IV
By Cheryl K. Gooden, MD
Mount Sinai Medical Center
New York, NY
The second afternoon session on Saturday moderated by Eugenie Heitmiller, MD (Johns Hopkins Hospital) provided an overview of the role of technology and how it interfaces with the safe delivery of anesthesia, how human factors engineering contributes to the design of safer medical devices, and how anesthesiologists can contribute to patient safety.
Matthew Weinger, MD (Vanderbilt University Medical Center) presented “Understanding the Role of Technology in Patient Safety.” He began by emphasizing the fact that most untoward events associated with the use of medical devices are the result of their poor design. This is in contrast to what one might think as a cause of the untoward events, mainly device failure. For example, he described the “unintentional misconnections,” whereby the end of the non-invasive blood pressure cable was connected to the IV catheter with heparin lock. The result for the patient was an air embolism. Further, Dr. Weinger highlighted the results of a prospective study involving approximately 800 anesthesia cases. The conclusion of the study was that one of the more common equipment-related events in the OR was linked to the OR table.
Technology is generally a good thing. Dr. Weinger presented several videos of cases in which technology related events were common. He posed the following question to the audience, as to “why doesn’t technology play nicely with clinicians?” He identified several reasons for why this is the case. First, it is necessary to examine the user interface. Second, there can be a failure to understand clinicians’ needs and requirements when we are working. Third, there can be a failure to appreciate the complexity of clinical care. Finally, there can be a failure to understand the context of use of the technology.
Dr. Weinger concluded his presentation with the facts that most technology is still not user friendly and user errors are a major contributor to anesthesia adverse events. Lastly, he encouraged the audience to remain vigilant of technology-induced failure modes and to seek better designs of the technology through our feedback.
Saturday, March 16 - Session V
By Jennifer Meyers, MD
Seattle Children’s Hospital
Seattle, WA
Allison Kinder Ross, MD (Duke University) began the session with a case description of a South African boy presenting for surgery with a medical history that included “allergy to all anesthetics.” Careful history and description revealed his mitochondrial disorder (MD) and the current lack of clarity among specialists for the safest anesthetic choices for this patient population.
Patients with known or suspected mitochondrial disorders usually first present for an anesthetic when they are referred for a muscle biopsy. Other common procedures address the wide variation in severity of organ dysfunction, including hearing tests such as Brainstem Auditory Evoked Responses (BAERs), MRI, upper GI, and endoscopy. Once diagnosed, many follow-up diagnostic and therapeutic interventions follow the course of this multi-system disease.
During preoperative evaluation, be sure to obtain detailed history of: increased aspiration risk and swallowing issues associated with gut immotility, hypotonia, developmental delay, and seizures. On laboratory evaluation, seventy to eighty percent of patients will have elevated serum lactate >3mm/L, and this finding is highly suggestive of a MD; creatine kinase may be normal or slightly elevated. Dr. Ross admonished: do not overlook the cardiac workup! Twenty percent of patients with mitochondrial disorders will have cardiomyopathy, and this subgroup carries a three times greater mortality rate compared to MD patients without cardiac issues.
Since this is a metabolic disorder of impaired energy production, minimizing the preoperative fasting period is imperative, often requiring glucose supplementation. During long procedures with expected fluid shifts, consider placing an arterial or central venous line to follow lactate and acid/base status, since these can vary unpredictably in patients with MD.
Dr. Ross reviewed the risk of Malignant Hyperthermia (MH) in these patients, first pointing out that MH involves the ryanodine receptor, which is NOT related to oxidative phosphorylation and MD. To the unfamiliar provider, many aspects may appear to overlap. Case reports of rhabdomyolysis that presented with clinical similarities to MH confuse the literature representation. In addition, both conditions are diagnosed by muscle biopsy, and MD patients may have abnormal halothane caffeine contracture test. Patients with complex 1 disorders are sensitive to Sevoflurane induction- achieving a markedly lower BIS than non-disordered patients with the same exposure of inhaled anesthetic. (Morgan article 2002 Anesth 96(5) 1268-70) Myoglobinuria may be an early sign of either MH or rhabdomyolysis. Dr. Kinder Ross recommends having a high index of suspicion, however nontriggering technique in not officially endorsed in any guidelines.
The most commonly used alternative to inhaled anesthetics is propofol which Dr. Kinder Ross pointed out, has a poor reputation in the lay press. In addition, several websites list the drug as “shown to impair mitochondrial function,” and this can cause considerable misinformation and confusion among caregivers. A subset of MD patients are known to develop Propofol Infusion Syndrome (PRIS) at lower doses due to the mechanism of action of the drug at oxidative channels. For additional reading, refer to: Metabolic Acidosis due to Propofol Infusion Farag et al Anesth 2005 102(3):
For perioperative pain management, all opioids are considered safe choices when dosed appropriately. Supplemental regional anesthesia is often utilized for muscle biopsies. Local anesthetics have been found to have a direct impact on oxidative phosphorylation, so care must be taken if/when selecting continuous infusion rates and choice of concentration. No conclusive studies or guidelines exist to date; Dr. Ross advises: “just be careful.”
Duke University has launched a new database and is looking for collaborative group members for the SPA Mitochondrial Disease SIG to participate in data collection. Send email inquiries to MitoGA@duke.edu if interested.
In summary, Dr. Ross provided some general common sense concepts to guide care of patients with mitochondrial disorders. Avoid increased energy use – maintain normothermia, achieve pain-free state as early as possible, and avoid acidosis. Avoid decreased energy supply-- minimize NPO period, PONV prophylaxis, provide supplemental glucose.
Next up, Anne Lynn, MD (Seattle Children’s Hospital) admirably stepped in for Alan Schwartz‘s last minute absence and provided an overview of updates in perioperative emergency care, including anaphylaxis, fasting guidelines, local anesthetic toxicity, pulmonary hypertension, and use of crisis checklists.
To begin with, she summarized World Allergy Organization anaphylaxis guidelines, highlighting the importance of pattern recognition. What to look for: cutaneous manifestations PLUS other features. Intramuscular delivery of epinephrine (0.01/kg IM in the anterior thigh) is recommended in published guidelines simply for consistency because intravenous access not available in all situations as it typically is for us in the perioperative setting.
Next, Dr. Lynn reviewed the literature on preoperative fasting guidelines, a subject which continues to have a paucity of scientific evidence since aspiration is such a rare occurrence. Although some weak studies have shown decreased gastric volume and/or acidity in specific patient populations, evidence does NOT support the routine use of medications alter the risk or severity of aspiration in healthy patients for elective procedures.
In the realm of local anesthetic toxicity, a task force in 2010 updated the 2001 ASRA guidelines by reviewing case series and animal studies on systemic toxicity (cardiac and CNS). Lipid emulsion (1.5 mL/kg 20% then 0.25 mL/kg infusion until stable for 10 min) remains the definitive treatment of choice, and Dr. Lynn pointed out that Propofol is NOT a substitute for intralipid! The primary method of prevention remains vigilant incremental aspiration and injection in fifteen second intervals. Of note, ultrasound guidance of regional block needles is yet unproven to alter the occurrence of systemic toxicity.
Dr. Lynn reviewed the pathophysiology and current treatment of neonatal pulmonary hypertension (NPH), most often due to prematurity-related chronic lung disease. NPH continues to result in 5-10% mortality plus significant morbidity (most commonly developmental delay) in survivors. Surfactant is no longer a universal recommendation in premature infants and only given if patients are deficient. Induced alkalosis? No longer! The effect is only transient. However, acidosis can significantly worsen symptoms and outcomes and should be treated aggressively. Sildenafil can sometimes be effective, though no specific guidelines exist. Only sixty percent of patients seem to respond to nitric oxide, and to varying extents.
Lastly, Dr. Lynn summarized a fantastic resource published by the American College of Surgeons in 2011: Crisis checklists for the OR. These are prioritized checklists of twelve critical perioperative events that occur infrequently but require swift identification and treatment, for example fire, hemorrhage, and failed airway Many hospitals have made laminated cards for bedside reference that are color-coded, visually simplified, single page, and tailored to each issue. A study demonstrated improvement in critical response during simulations scenarios when providers had access to the checklist cards. An audience member pointed out that our own SPA is developing pediatric-specific critical events checklists which are now available on the website. Please send feedback through the Quality and Safety Committee when you use them!
Sunday, March 17 - Session I
By Kim Nesbitt, MD
Vanderbilt Children’s Hospital
Nashville, TN
The final day of the SPA meeting included Editor’s Best Picks, moderated by Dr. Jeffrey Morray (Phoenix Children’s Hospital).
This session began with Dr. J. Lance Lichtor (Yale-New Haven Children’s Hospital) presenting, from Anesthesiology, his favorite three 2012 pediatric anesthesiology articles. He first discussed an article from the July issue, Volume 117 entitled Effect of Nitrous Oxide Exposure during Surgery on the Homocysteine Concentrations of Children. Pichardo, et al¸ reported the effects of nitrous oxide anesthesia on total plasma homocysteine (tHcy) by measuring these levels the morning after surgery. The authors investigated the effect of nitrous oxide anesthesia on the plasma tHcy concentrations in children the morning after surgery. They also looked at whether blood concentrations of folate and vitamins B12 and B6 were associated with any potential increase.
His next choice was the study by Simpao et al, December 2012, 117(6), entitled Cephalad Migration of Pediatric Caudal Catheters with Change from Prone to Supine Position. Lastly, he noted Epidermolysis Bullosa in the Newborn. This case report submitted from Anthony Tsui MBBS, addressed the challenging management of a 2kg, 2 day old neonate for Hickman placement and pyloromyotomy.
Dr. Peter Davis (Children’s Hospital of Pittsburgh), section editor for pediatrics, presented his selections from Anesthesia & Analgesia. Are Our Children All Right? by Mifflin, Hackman, and Chorney, (A&A 2012, volume 115, pages 1162-7) looked at the effect on anxiety of streamed video clips for distraction during inhalation induction. Dr. Davis noted that technology can enhance our efforts to distract. This being the most effective method of alleviating anxiety, as was so clearly reported by Zeev Kain et al, in A&A 1998:88-102.
The second notable pick was a prospective study, by D Polaner, A Taenzer and B Walker, et al, published in A&A December 2012 ;115(6):1353-64. Pediatric Regional Anesthesia Network (PRAN): A Multi-Institutional Study of the Use and Incidence of Pediatric Regional Anesthesia, produced results through a consortium of 6 core centers, and 14 other participating centers. He pointed out that the data was consistent with the European studies and showed a low overall incidence of complications; however those that were found were noted to be devastating and costly from a medico-legal perspective.
The third article recommended by Dr. Davis continues the look at neural blockade and complications. Neurological Complications Associated with Epidural Analgesia in Children: A Report of 4 Cases of Ambiguous Etiologies (2012; 6:1365-70). Meyer, Krane, Goldschneider and Klein, discussed four case reports. Three of the four, resulted in sizeable settlements for resultant complications. Four cases of long term or permanent neurologic complications associated with epidural analgesia, and the possible mechanisms of injury, as well as implications for practice were discussed. Dr. Davis concluded his time on the platform with an invitation and open call for academicians interested in taking over his position as A & A’s section editor. He requested CVs be sent to him for review, as he recruits an appropriate replacement to step in to follow his notable 10 years of dedicated service.
Pediatric Anesthesia’s section editor, Dr. Charles Cote (Massachusetts General Hospital) presented many of his favorite articles published in 2012 and early 2013. Though too numerous to mention them all individually, identifying volume citation information has been included for further reference. He started with an Isabelle Murat tribute from June, the publication of IASR proceedings, and an article in the themed issue with Per-Arne Lonnqvist as guest editor, entitled Pediatric Regional Anesthesia—Starting 2012 with a Bang. He continued with three “must reads”, from Volume 22 (pp 530-538, 539-552 and 627). A “fun paper” was noted by him and can be found in 22:288-296. Three “Quick hits” were discussed and involved CO oximetry (22:859-864), comparison of standard technique in IV cannulation with the AccuVein AV300 (22:884-889), and vital sign change with intravenous ropivacaine from 2013 (23:144-148).
He quickly discussed two papers dealing with controversial issues. The first was a paper out of Lebanon discussing their studies involving intubation with and without adjunct therapy versus muscle relaxation. The second paper by Weiss et al in the February issue (2013, pp. 103-110) was a study entitled Endoscopic airway findings in children with or without prior endotracheal intubation, which showed an increase in granuloma development following endotracheal intubation. He concluded with a quick survey of topics of interest with his choice for related articles addressing the studies and findings. Topics included two papers discussing congenital heart disease found on pages 647-653 and 865-870 from Volume 22. Other papers he identified due to them being related to high interest topics including fasting guidelines and narcotics therapy for T&A surgery.
Completing the Editor’s Best Picks session, the three editors participated in a lively panel and audience question and answer period. Neurotoxicity of anesthesia in the developing brain was a heavily discussed topic with significant input from the audience including comments from Dr. Jerry Parness and Dr. Randall Flick. The majority consensus was in agreement that the subject needed further study with more specific and relevant research.
SPA President Dr. Nancy Glass, addressed the audience and expressed her appreciation for their participation in the 2013 Winter Meeting and gave a warm word of gratitude for the presenters and faculty who had supported the meeting and society with their work and efforts. Special credit and appreciation was given to the outstanding SPA staff for their efforts in making our meeting excellent.
She then turned the focus to the European Congress on Paediatric Anesthesia (ESPA-SPA) meeting, in Spain, September 5th-7th. She appealed to all interested parties interested in submitting abstracts to note the June 15th deadline.
The Spring Meeting of the Society for Pediatric Anesthesia concluded with a lively and engaging session of Jeopardy, with the ever popular and entertaining Dr. Myron Yaster. The audience participation questions covered variety of subjects related to our clinical practices and specialty preferences. Click here if you are interested in a review of the audience responses.
Of special interest were questions generated by the Communications Committee for SPA in an effort to assure that our direction and efforts were in line with the needs of society members. The effectiveness and utilization of our website was also addressed in regard to making the site an information resource for patient. The overwhelming feedback, from the SPA audience in attendance was that it should be an available resource for the public and should provide information and education about anesthesia care for the pediatric patient.
Peds Passport
 By Helen V. Lauro, MD, MPH, MSEd, FAAP
By Helen V. Lauro, MD, MPH, MSEd, FAAP
NOTE: Purchase of travel insurance with air evacuation coverage is highly recommended to meeting attendees and their families
2013
July 11-14: Myrtle Beach, South Carolina, USA
Pediatric Anesthesia Update
Tel: (800)-222-6927
Information: Northwest American Seminars, P.O. Box 2797, Pasco, WA 99302
Website: http://www.nwas.com
Email: info@nwas.com
August 22-25: Niagara Falls, Ontario, Canada
Anesthesia Update: Emphasis on Pediatrics
Tel: (800)-222-6927
Information: Northwest American Seminars, P.O. Box 2797, Pasco, WA 99302
Website: http://www.nwas.com
Email: info@nwas.com
August 23-25: Rosemont, Illinois, USA
Intensive Review of Pediatric Anesthesia
Tel: (804)-282-9780, Fax (804)-282-0900
Information: Society of Pediatric Anesthesia, 2209 Dickens Road, Richmond, VA 23230-2005
Website: http://www.pedsanesthesia.org
Email: spa@societyhq.com
September 5-7: Geneva, Switzerland
Annual Congress of the European Society for Paediatric Anaesthesiology: Geneva 2013
Tel: +31 (0) 887555555; Fax: +31 (0) 887554702
Information: Secretariat ESPA, c/o Department of Anesthesia, Intensive Care and Emergency Medicine, Wilhelmina Children Hospital/University Medical centre Utrecht Lundlaan 6, Mail stop: KG 02.307.1, PoBox 85090, 3508 AB Utrecht, The Netherlands
Website: http://www.euroespa.org
Email: secretary@euroespa.org
September 18-21: Lucerne, Switzerland
3rd European Conference on Pediatric and Neonatal Cardiac Intensive Care
Tel: + 41 (0)22 839 8484
Information: Symporg SA, Rue Rousseau 30, 1201 Geneva, Switzerland
Website: http://www.symporg.com
Email: elaget@symporg.com
September 25-27: London, United Kingdom
27th Paediatric Intensive Care Society Conference (PICS)
Tel: +44 (0) 207 383 8041
Information: Conference Secretariat, Kenes UK, The Euston Office, One Euston Square, 40 Melton Street, London, NW1 2 FD
Website: http://picsmeeting.com
Email: pics@kenes.com
October 11: San Francisco, California, USA
Society of Pediatric Anesthesia (SPA) 27th Annual Meeting
Tel: (804)-282-9780, Fax (804)-282-0900
Information: Society of Pediatric Anesthesia, 2209 Dickens Road, Richmond, VA 23230-2005
Website: http://www.pedsanesthesia.org
Email: spa@societyhq.com
October 16 – 17, 2013: Port of Spain, Trinidad and Tobago
World Congress of Surgery, Obstetrics, Trauma and Anesthesia
Tel: (410) 614-0149
Information: Laura Friend, CME Coordinator
Johns Hopkins University SOM, Office of Continuing Medical Education
720 Rutland Avenue, Turner 20, Baltimore, MD 21205-2195
Email: worldcongress@jhmi.edu
Website: www.wcsota.com
October 17-20: Manly, New South Wales (NSW), Australia
Society for Paediatric Anaesthesia in New Zealand and Australia (SPANZA) Conference 2013
Tel: +61 2 4973 6573; Fax: +61 2 4973 6609
Information: SPANZA Secretariat, P.O. Box 180, Morriset, New South Wales, Australia 2264
Email: spanza@willorganise.com.au
Website: http://www.spanza.org.au/index.php/news-events/events
November 8-10: Toronto, Ontario, Canada
26th Pediatric Anesthesia Conference
Tel: (416)-813-7654 ext. 28120 (Ms. Michelle Wan) or (416)-813-7445 (Ms. Shue Lin Loo)
Information: Dept. of Anesthesia and Pain Medicine, The Hospital for Sick Children, 555 University Avenue, Toronto, Canada MSG 1X8
Email: li.conferences@sickids.ca, pediatric.anesthesia@sickkids.ca
Website” http://www.cvent.com/events/pediatric-anesthesia-conference/event-summary-916d4a5d9bc942a09178bd7ad27c102a.aspx
November 8-10: Durban, South Africa
17th Annual Paediatric Congress of South Africa
Tel: 031 539 3605/082 553 8201; Fax: 0866 179 423
Information: Ms. Alison Shaw, Conference Secretariat
Website: http://www.pacsa2013.co.za
Email: alison@royalh.co.za
2014
March 6: Fort Lauderdale, Florida, USA
Congenital Cardiac Anesthesia Society (CCAS) 2014 Meeting
Tel: (804)-282-9780, Fax (804)-282-0900
Information: Congenital Cardiac Anesthesia Society, 2209 Dickens Road, Richmond, VA 23230-2005
Website: http://www.ccasociety.org
March 6: Fort Lauderdale, Florida, USA
Society for Pediatric Pain Medicine (SPPM) 2014 Meeting
Tel: (804)-282-9780, Fax (804)-282-0900
Information: Society for Pediatric Pain Medicine, 2209 Dickens Road, Richmond, VA 23230-2005
Website: http://www.pedsanesthesia.org
March 6-9: Fort Lauderdale, Florida, USA
Society of Pediatric Anesthesia (SPA)/American Association of Pediatrics (AAP) 2014 Winter Meeting
Tel: (804)-282-9780, Fax (804)-282-0900
Information: Society of Pediatric Anesthesia, 2209 Dickens Road, Richmond, VA 23230-2005
Website: http://www.pedsanesthesia.org
May 4-7: Istanbul, Turkey
7th World Congress on Pediatric Intensive and Critical Care (PICC 2014)
Tel: +41 22 9080488., Fax: +41 22 9069140
Information: Kenes International Ltd, 1-3 Rue de Chantepoulet, P.O. Box 1726, CH-1211, Geneva 1, Switzerland
Website: http://www.kenes.com
Email: info@kenes.com
May 8-11: Istanbul, Turkey
12th Asian Society of Paediatric Anaesthesiologist Congress
Information: ASPA Secretariat, Department of Paediatric Anaesthesia, KK Women’s and Children’s Hospital, 100 Bukit Timah Road, Singapore 229899
Website: http://www.aspa-2000.com
Email: SERPOZGEN@TNN.NET
May 15-16: Leeds, United Kingdom
Association of Paediatric Anaesthetists of Great Britain and Ireland Meeting
Tel: +44 (0) 20 7631 8887, Fax: +44 (0) 20 76314352
Information: APA Administration Office, AAGBI, 21 Portland Place, London, W1B 1YP, United Kingdom
Website: http://www.apagbi.org.uk
Email: apagviadministration@aagbi.org
June 12-15: Key West, Florida, United States
8th Annual Pediatric Anesthesia Review
Tel: (800)-222-6927
Information: Northwest American Seminars, P.O. Box 2797, Pasco, WA 99302
Website: http://www.nwas.com
Email: info@nwas.com
October 17-21: Barcelona, Spain
25th Annual Congress of European Society of Paediatric and Neonatal Intensive Care (ESPNIC) in conjunction with the 5th Congress of the European Academy of Paediatric Societies (EAPS)
Tel: +41 22 9080488., Fax: +41 22 9069140
Information: Kenes International Ltd, 1-3 Rue de Chantepoulet, P.O. Box 1726, CH-1211, Geneva 1, Switzerland
Website: http://www.kenes.com
Email: paediatrics@kenes.com
Footnote:
Please forward all information concerning congresses relevant to
Pediatric Anesthesia to:
Helen V. Lauro, MD, MPH, MSEd, FAAP, SUNY Downstate Medical Center University Hospital of Brooklyn at Long Island College Hospital, Department of Anesthesiology, 339 Hicks Street, Brooklyn, New York 11201.




