Annual Meeting Reviews
Session IV: Anesthesia On the Road Again
Reviewed by Kamie Yang, MD
C.S. Mott Children’s Hospital
Sedation Service: Anesthesia vs. Other Specialists
This session discussed who should provide pediatric sedation services outside of the operating room – anesthesiologists or non-anesthesiologists?
Samuel D. Yanofsky MD, MSEd (Children’s Hospital of Los Angeles) argued that anesthesiologists should be the soul providers of pediatric sedation services outside of the operating room. Ironically, in the 2000’s, the US was far behind Europe and provided minimal off-site sedation services. During this time, surveys of oncology centers indicated that bone marrow biopsies were done without sedation 50% of the time in the US as compared with 0% of the time in Europe. However, in the last 10 years, the demand for pediatric sedation services has exploded in the US and sedation is now being utilized for everything from radiology imaging, dentistry, radiation oncology treatments, and inpatient medical services.
A wide variety of clinicians currently provide pediatric sedation services outside of the operating room. These include (1) Direct Physician Care (Anesthesiologist, Critical Care Physician, Emergency Medicine Physician, Radiologist (2) Other Providers (Nurse Practitioners, Radiology Nurses, Physician Assistants and (3) Sedation Teams with mixed provider types.
With goals of increasing safety, non-anesthesiologist sedation practitioners are often limited to administering 1 medication. These providers are thus often left administering less safe, less effective, and longer acting medications. Non-anesthesia providers also often lack essential airway skills to rescue from deeper levels of sedation, undoubtedly increasing the potential for adverse events.
Dr. Yanofsky continued to discuss a prospective, observational study published in 1997 of 1140 children who were sedated by non-anesthesiologists. The adverse event rate was noted to be high in this study, with 5.3% of cases resulting in adverse respiratory events. Additionally, 13% of cases were also inadequately sedated.
Dr. Yanofsky concluded his talk by arguing that anesthesiologists are most suited for providing all pediatric sedation services. We understand the spectrum of issues that must be considered during the preoperative, intraoperative, and postoperative period and don’t merely aim to get a given patient through their procedure. We also understand the importance of formulating back-up plans, the impact of complex systems issues, and are capable of being efficient while still maintaining safety. Finally, since sedation services are needed throughout any pediatric hospital, anesthesiologist-performed sedation has the added benefit of inherently increasing the department’s perceived value within their institutions.
Randall C. Wetzel, MBBS, FAAP (Children’s Hospital of Los Angeles) countered that anesthesiologists must embrace the idea that non-anesthesiologists will be providing pediatric sedation services outside of the operating room and that the best solution to manage this inevitability is to provide a system of coherent anesthesia oversight.
Dr. Wetzel continued to discuss how his own institution, the Children’s Hospital of Los Angeles (CHLA), utilizes a Sedation Team that is overseen by the Department of Anesthesiology. Called the Non-Anesthesiologist Pediatric Sedation Service (NAPS), the team consists of PALS and APLS certified pediatricians with prior airway skills and includes Pediatric Emergency Department Physicians and Pediatric Critical Care Intensivists. They are trained to provide deep sedation with propofol or ketamine to children undergoing procedures outside of the operating room. Specifically, their sedation-specific training consists of the following:
- 20 hours of didactic education and self guided learning
- 20 hours of directly supervised apprenticeship with an anesthesiologist
- Successful completion of a written test
Interestingly, he noted that one important element of training these physicians is to change their “way of thinking”. While Emergency Department Physicians and Intensivists are often presented with handling a resuscitation on a coding patient, he teaches his trainees that their goal is no longer to find ways “to get out of trouble”, but to find ways “to stay out of trouble.”
Dr. Wetzel continued to discuss his own, non-published results from an observational study he performed comparing the safety of NAPS Sedation to sedation services provided by an anesthesiologist. In this study, he compared the complication rates seen between non-anesthesiologists to anesthesiologists performing propofol sedation in 381 infants (2 weeks - 1 year). The sicker, smaller, and younger patients received care from anesthesiologists. No deaths, arrests, hospitalizations, or intubations were seen. The complication rate (need for fluid, atropine, BVM, nasal trumpet) was higher in the group treated by anesthesiologists (12.5% vs. 6.5%). From this data, he did not conclude that the anesthesiologist group provided less safe care, but that the NAPS Service selection process worked well and assigned the sicker patients to anesthesiologists.
At CHLA, NAPS Service Pediatricians are credentialed like anesthesiologists (independent anesthesia providers, but not autonomous) to provide general anesthesia services and are thus able to bill and are insured as anesthesiologists. Thus, CHLA was not only able to extended their anesthesia services to make the hospital, patients, radiologists and anesthesiologists happy, they also increased their revenues by > $150k per year.
He then continued by discussing a landmark study that showed that non-anesthesiologist Pediatric Sedation Teams were a safe alternative. Published in 2009 from the Pediatric Sedation Research Consortium, this multicenter prospective study researched the complications seen in 49,836 propofol sedation/anesthesia encounters. Anesthesiologists, Emergency Department Physicians and Intensivists made up 95% of the practitioners administering the sedation regimens. The complications rates were similar to anesthesia services for general anesthesia (arrests, death, and aspiration). Though this study defines the current standards for safety in pediatric sedation, Dr. Wetzel emphasized that these favorable results were seen because of two important reasons:
- The study included physicians with airway and cardiovascular skills and did not include radiologists, nurses, or advanced practice nurses.
- The physicians in this study were working within an established system that included a willingness to report complications and utilize this information to continuously work to improve care.
More importantly, he warned against construing this report as indicating that propofol anesthesia administered by any practitioner is universally safe. Instead, favorable results were seen in this study because there were strong anesthesia systems of care in place. He argued that it is essential that these sedation systems be overseen by Anesthesiology Departments because we have the unique expertise and knowledge to best manage these systems.
Dr. Wetzel then closed his interesting talk by highlighting the steps needed to create a coherent system of anesthesia oversight for sedation services. In his view, adequate oversight should include the following seven elements:
- Define the knowledge and skills required.
- Define the training required and oversee it.
- Assure the sites where anesthesia is practiced meet recognized standards.
- Credential all providers.
- Assure maintenance of skills.
- Perform practice review and continuous quality improvement.
- Perform research.
Too Hot to Handle: Photons, Protons, & MIBG
Dr. Dawit T. Haile MD (Mayo Clinic Children’s Center) opened his talk by discussing radionuclide imaging and therapy with MIBG. MIBG is an abbreviation for meta-iodobenzylguanidine. These compounds are injected intravenously and are radiolabeled with 123I or 131I. MIBG is avidly taken up by actively dividing cells, allowing for both tumor imaging and cancer therapy. MIBG scans differ from traditional CT or MRI scans because they highlight areas of functional activity, thus distinguishing residual tumor from inactive, post-therapy remains. Drugs that interfere with MIBG include calcium channel blockers, ACE inhibitors, adrenergic receptor blockers, amiodarone, and sodium pump inhibitors like digoxin. Once injected, the patient and their urine are radioactive for 4 days, as the drug is renally excreted.
Dr. Haile then continued to discuss external radiotherapy. External radiotherapy cures 85% of pediatric cancer patients. However, 65% of survivors have chronic health conditions and 20% die of a second malignancy or treatment-related event. It is known that conventional external radiation with traditional photons results in lower IQ scores, with 50% of patients developing below normal IQs. [Dev Med Child Neurol. 2013;55(5):408]. For this reason, though up-front radiation correlates with better survival, it is sometimes differed because of the collateral injury to normal brain that is inevitably seen following treatment.
In the past, charged particle external radiotherapy was traditionally done with photons. However, more recently, protons are being utilized. These compounds differ in that photons emit diffuse beams of radioactivity that result in significant normal tissue damage, while newer protons can more specifically target the tumor and limit collateral damage. The precision of proton therapy can be so exact as to be “pencil point”, therefore making patient immobilization absolutely essential to optimizing calculations and the accuracy of treatment.
Proton external radiotherapy treatment usually entails immobilization with a full-faced hard mask that is often not well tolerated by children < 8 years old. Treatments are daily or twice a day and can last for 2 to 4 weeks. Due to the frequency of treatments, airway manipulation is often avoided and optimal nausea and vomiting prophylaxis is pursued. NPO guidelines are also altered and high protein/calorie clear liquids are allowed 2 hours prior to the procedure.
Currently, most institutions provide propofol general anesthesia with a natural airway for these procedures. Propofol infusions have been shown to yield satisfactory treatment conditions 98% of the time [Int. J. Radiation Oncology Biol. Phys. 2001; 49 (3): 771]. When high doses of propofol are needed, Dr. Haile adds dexmedetomidine as his second agent and avoids narcotics to minimize nausea and vomiting. At his institution, airway manipulation with ETT or LMA remains rare.
Proton treatment is about 2.5 times more expensive than photon treatment ($13.5K vs. $5.6K), but leads to less adverse effects (with estimated overall costs of $5.6K vs. $45K over time) that outweigh the initial increase in cost. Other long-term benefits of proton beam treatment over traditional photon therapy include a decreased incidence of secondary malignancy, (5.2% vs. 7.5%), no decrease in IQ, and a better quality of life post-treatment. [Into J Radiation Oncol Biol Phys, 2013 87(1)46e; Radiat. Oncol. Biol. Phys. 2015. 93, 400–407; York et al Radiother Oncol. 2014 Oct;113(1):89-94].
There are currently 24 proton beam centers in the country and 11 more are being constructed. At the Mayo Clinic, the Anesthesia and Radiation Oncology Departments collaborated extensively to plan construction of the external beam treatment area. The area includes 2 induction rooms, 4 pre/post anesthesia rooms, and 4 gantry areas. Ultimately, Dr. Haile’s goal is to provide optimal workflow so as not to interfere with productivity and increase overall costs. He ultimately aims to limit a patient’s time in the treatment room and make “pediatric treatment room time” equal to “adult treatment room time”.
Dr. Haile then described the typical workflow: the patient is induced on a stretcher (called a “couch”) in the induction area, the immobilizer attached, NC placed, and monitors attached. All monitors and infusions remain attached to the “couch”. The “couch” is then taken to the radiation treatment area by a robotic arm. The oxygen is attached to a port on the robotic arm so that no tubing is left hanging outside of the “couch” and treatment commences.
Dr. Haile ended his talk by describing the risks of external beam radiation to providers. The radiation treatment room is extensively shielded and unlike CT, the beam automatically shuts off when the door is opened, so inadvertent exposure is impossible. Of note, radioactive neutrons particles remain near the patient after the beam is turned off, but these particles remain active for only a few minutes. Interestingly, these neutron particles can adversely affect ICDs and pacemakers, resetting them to factory settings and may contraindicate proton beam treatment for some patients that are dependent on these devices.
Cath Lab Consensus Statement
Procedures performed in pediatric cardiac catheterization labs have transitioned from diagnostic to interventional and therapeutic. The current practice of sedation and anesthesia varies greatly among institutions, largely based on program size and personnel resources. Thus, a multi-society expert panel (including Society for Cardiovascular Angiography and Interventions (SCAI), Congenital Cardiac Anesthesia Society (CCAS), and the Society of Pediatric Anesthesia (SPA)) convened to provide practitioners and institutions with guidance on anesthetic techniques and personnel necessary to safely perform these procedures.
Dr. Rahul Baijal MD (Texas Children’s Hospital, Houston) summarized the most salient data that went into creating this consensus statement. However, he cautioned that it is not uncommon to see a widely heterogeneous patient population in the pediatric catheterization laboratory. Patients vary in age (from neonate to adult), complexity of their physiologic lesions, and level of ability to cooperate. Thus, Dr. Baijal emphasized that the guidelines put forth by this expert panel could not encompass all clinical circumstances and cannot replace clinical judgment.
Dr. Baijal opened by discussing how peri-procedural complications seen in the cardiac catheterization lab can be related to the (1) procedure, (2) patient, and (3) practitioner/anesthesia.
Procedure-related risks for adverse events include arrhythmias (often transient), vascular and cardiac damage, thrombosis/thromboembolism, valve incompetence, allergy to contrast/medications, and neurologic injury.
He then continued to discuss patient-related risks for adverse events. Children with congenital heart disease (CHD) have long been known to be at increased risks for adverse events and cardiac arrest during non-cardiac surgery. According to Murat et al (2004), Braz et al (2006), and Flick et al (2009), risk factors include:
- Age < 1 year
- Need for pre-procedural intubation
- Higher ASA score
- Emergency procedures with GA
Dr. Baijal then continued to discuss a study recently published in October 2016. Utilizing the American College of Surgeons National Surgical Quality Improvement Program database, Faraoni et al looked at 4375 children with CHD receiving non-cardiac surgery. Overall in-hospital mortality was found to be 4.7% (204/4375). In this study, predictors for in-hospital mortality included:
- Emergency procedure
- Type of lesion:
a. Severe CHD (RVOTO, LVOTO, pulmonary hypertension, myocardial derangement)
b. Single-ventricle physiology - Markers of critical illness
a. Previous surgery within 30 days
b. Preoperative inotropic support
c. Preoperative cardiopulmonary resuscitation
d. Acute or chronic kidney injury
e. Preoperative mechanical ventilation prior to non-cardiac surgery
Next, Dr. Baijal turned to discussing the Pediatric Perioperative Cardiac Arrest Registry (POCA). Published in 2010, this registry collected perioperative data on pediatric cardiac arrests from 80 North American institutions over a 9-year period. A total of 373 cardiac arrests were documented in this registry. He highlighted the following notable data points from this study:
- 34% (127/373) of cardiac arrests were seen in patients with CHD.
- Mortality was higher if patient had CHD (33%) than in patients without (23%).
- 17% of cardiac arrests occurred in the cardiac catheterization laboratory.
- Younger patients were more likely to have a cardiac arrest. (½ less than 6 months of age, 70% less than 2 years of age).
- Most common CHD lesion: Single ventricle (24 patients).
- Patients with CHD were: unrepaired (59%) or palliated (26%) at time of cardiac arrest.
- Lesions associated with highest mortality rates: Aortic Stenosis (62%) and Cardiomyopathy (50%).
As seen in studies of non-cardiac surgery, children with CHD are also at high risk for adverse events in the cardiac catheterization laboratory. Vitello et al. (1998) and Bennet et al (2005) estimated that children with CHD have an 8-11% risk of adverse events. In these studies, more adverse events were seen with interventional (rather than diagnostic) procedures and in children 6-12 months of age.
More recently, Odegard et al. (2014) evaluated over 7000 cardiac catheterization procedures at a single, large pediatric tertiary referral center. Cardiac arrest occurred in 0.96% of procedures and was again associated with:
- Interventional procedures (VSD device closure, intervention for intact atrial septum, mitral valve dilation, pulmonary vein dilation, and pulmonary artery dilation)
- Patients < 1 year of age
Most importantly, Dr. Baijal highlighted that in this study, there was a reduction in cardiac arrests from 1.5 to 0.7 per 100 procedures as cardiac anesthesia staff involvement increased. This improvement was seen as the author’s institution moved away from utilizing nurse-administered sedation toward involving cardiac anesthesia personnel in all aspects of patient management, including scheduling, selecting the anesthetic technique, and anticipating potential adverse events.
Children with pulmonary arterial hypertension are also at increased risk of cardiac arrest in the catheterization lab. According to Carmosino et al., these patients have a 4-5% risk of cardiac arrest and possessing a baseline suprasystemic RV pressure was a significant predictor of major complications. However, in this study, risk for cardiac arrest was not associated with patient age, etiology of pulmonary hypertension, type of anesthetic, or airway management. Similarly, Taylor et al. found a 5.7% rate of cardiac arrest in the catheterization lab among patients with pulmonary hypertension. Risk factors for cardiac arrest included a history of syncope, dizziness, or chest pain and a tricuspid regurgitant jet > 4m/s.
Dr. Baijal continued to discussed three pediatric cardiac catheterization registries that further worked to risk-stratify cases utilizing patient and procedural factors. These registries included:
- Congenital Cardiac Catheterization Outcomes Project (C3PO) (CHARM score)
- Improving Pediatric and Adult Congenital Treatments (IMPACT)
- Congenital Cardiac Interventional Study Consortium (CRISP score)
The Congenital Cardiac Catheterization Outcomes Project (C3PO) gathered prospective data over a 3-year period and included over 13483 cardiac catheterizations from 8 institutions. Patient and procedural characteristics were collected and all adverse events were recorded. This data set was then utilized to create a risk model that would take into account variations in case-mix complexity between institutions.
From this data, each procedure was assigned a:
- Procedural Type Risk Score: Estimated the degree of interventional risk inherent to the procedure (0-5 points)
- Hemodynamic Vulnerability Score: Number of concerning hemodynamic indicators seen during the catheterization itself (not previously obtained, pre-procedure data) (0-2 points)
- Age Score: < 1year or > 1 year (0-1 point)
From these procedure and patient categorical data, a multivariable logistic regression model was developed to predict high-severity adverse events. Called the Congenital Heart Adjustment for Risk Method (CHARM), this regression model identified three independent variables that were associated with increased risk.
- Higher Procedural Type Risk score
- Hemodynamic Vulnerability Score > 2 risk factors (Risk factors: high systemic ventricular end-diastolic pressure, low arterial saturation, low mixed venous saturation, and high pulmonary artery systolic pressure)
- Age < 1year
In this registry, 2.1% of procedures were associated with life-threatening events and mortality was 0.28%.
From a second, expanded data set from 50 institutions and 19,608 pediatric catheterizations, The IMproving Pediatric and Adult Congenital Treatments (IMPACT) study also produced similar results. Though the cardiac arrest rate was higher at 0.8%, a similar rate of major adverse events (1.9%) was seen. Similar patient and procedural factors were also associated with increased risks of adverse events (including age <1 year, pre-procedure renal insufficiency, single-ventricle physiology, high procedure-type risk, low systemic saturation, low mixed venous saturation, high systemic ventricular end-diastolic pressure, high mean pulmonary artery pressure).
Dr. Baijal then discussed a third pediatric cardiac catheterization registry gathered by the Congenital Cardiac Interventional Study Consortium (CCISC). This group sought not only to account for case-mix differences between institutions, but unlike the previous two registries, also aimed to pre-procedurally stratify patients based on their individual physiology and procedural differences.
This registry included 18,564 procedures from 20 centers. Patient and procedure data was divided into 8 categories. Sub-components within each category were assigned a point value from 0 to 3. These point values were non-uniformly distributed within each of the 8 groups. The total of these point values was summarized as the Catheterization RISk Score for Pediatrics (CRISP) Score. The 8 data categories summed into the CRISP Score included the following:
- Age (< 30 days, 30 days to <1 year, >1 year)
- Weight (<2.5kg, 2.5-5kg, >5kg)
- Preoperative Inotropic Support (Non, yes/stable, yes/unstable)
- Systemic Illness/Organ Failure (None, Medically controlled/1 organ failure, Uncontrolled/>1 organ failure)
- Physiologic Category (As determined by prior studies, including systemic arterial saturation, PVR, ratio of RVSP/SBP, preoperative anemia electively or urgently treated, dynamic sub-pulmonary obstruction, significant AV valve regurgitation for the systemic ventricle)
- Pre-Catheterization Diagnosis
- Procedure Category
- Procedure Type (Diagnostic, Interventional, Hybrid)
In this cohort, as the CRISP score increased from 0 to 15 or more, the rate of serious adverse events dramatically increased from 1% to 36.8%.
Dr. Baijal then moved on to discuss the effects of an institution’s cardiac catheterization case volume on adverse events rates. O’Byrne et al studied a registry of 63,994 cardiac catheterizations from 38 centers. Not unexpectedly, the risk of death and need for post-procedure mechanical circulatory support decreased with increasing case volumes. Additional factors that were associated with these unfavorable endpoints included: younger age at catheterization (< 1 year), previous cardiac operation during the same hospital admission, pre-procedural need for vasoactive medications, and pre-procedural need for hemodialysis.
Moving onto the third determinant of peri-procedural complications in the cardiac catheterization lab, Dr. Baijal then discussed practitioner and anesthesia management issues. Overall, aesthetic management in the catheterization lab is not standardized. Instead, to formulate a reasonable anesthetic technique, the practitioner must have a good understanding of the patient’s baseline pathophysiology, the procedure being performed, the anesthesia-induced changes in hemodynamic parameters and respiratory mechanics, and the expected physiology after the procedure is complete.
Anesthetic agents are known to have a variety of effects on cardiac parameters. Dr. Baijal cautioned that all oxygen, volume loads (saline or blood transfusion), and vasopressor administration be communicated to the cardiologist because they can change cardiac output calculations, vascular resistance measurements, and filling pressures.
In terms of volume management, hypovolemia should be avoided especially in young, cyanotic, erythrocytotic patients and in shunt-dependent patients. Volume overload is also common during catheterization procedures, so in light of the unknown amount of volume that is given by the cardiologist, it is important for anesthesia practitioners to be judicious with their fluid management throughout these cases.
An initial hemoglobin level should be obtained to evaluate the severity of baseline erythrocytosis. Progressive anemia is also common during prolonged catheterization procedures due to frequent blood samplings, hemodilution, and inadvertent blood loss around vascular puncture sites. Anemia is poorly tolerated in cyanotic patients, so it is important to maintain hematocrits > 40. Also, anemia can elevate trans-stenotic pressure gradients, affecting catheterization results and the cardiologist’s overall decision making.
There is no preferred ventilation strategy in the catheterization laboratory. However, it is important to note that positive pressure ventilation can affect hemodynamic measurements, especially in patients with RV dysfunction, hypovolemia, and Fontan physiology. Also, though spontaneous ventilation allows for the maintenance of natural intrathoracic pressures, oversedation and airway obstruction can alter PVR and other measurements as well. Notably, in patients with pulmonary hypertension undergoing diagnostic catheterization, general anesthesia and sedation have been shown to have similar rates of adverse events.
The cardiac catheterization lab is a unique environment because it is often remote from other anesthetizing locations and access to the patient is often physically limited due to radiology equipment. Also, depending on the institution, mechanical circulatory support or cardiac surgery personnel may also not be readily available. Therefore, due to the complexity of the patients and cases performed in the catheterization lab, extensive systems must be in place. Dr. Baijal emphasized that communication between cardiologists, anesthesiologists, cardiac surgeons, and cardiac intensivists is of upmost importance. As such, specific periprocedureal checklists were also highlighted in this consensus statement and specific pre, intra, and post-procedure elements were discussed.
To close his summary of this consensus statement, Dr. Baijal addressed the question of who should be performing anesthesia for these cases. For both the pediatric cardiologist and the pediatric anesthesiologist, there is no formal sub-specialty training that is required prior to treating patients in the catheterization laboratory. Though discussions about offering advanced second-year fellowship training for pediatric anesthesiologists who want to work in this setting have started, there are a limited number of institutions actually providing this training. Also, with the current caseloads, it is currently simply not feasible to have a pediatric cardiac anesthesiologist providing care for all patients in the catheterization laboratory.
Thus, the SCAI/CCAS/SPA Expert Consensus Statement recommended stratifying patients based on CRISP scores and recommended a minimal level of anesthesia provider expertise for various CRISP levels as follows:
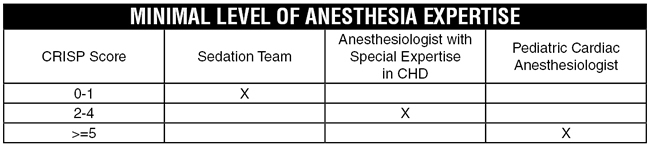
Dr. Baijal highlighted that in addition to this risk-stratification strategy, he generally considers high-risk patients and high-risk procedures to include the following: Patients with Williams syndrome, hypertrophic cardiomyopathy, single ventricle physiology, AV stenosis, MV stenosis, pulmonary hypertension, <1 year age, s/p heart transplant with coronary artery disease, pulmonary vein dilation, pulmonary artery dilation, VSD device closures, and balloon atrial septostomies.
In light of all these recommendations, Dr. Baijal closed his talk by re-emphasizing that the writers of this consensus statement ultimately did not want to limit small and medium sized programs from performing pediatric procedures in the catheterization lab. Instead, they aimed to provide guidance so that smaller institutions can assess and manage their overall risks based on their individual case mix and evolving patient and procedural related complexity.
References
- Hain RD, Campbell C. Invasive procedures carried out in conscious children: contrast between North America and Europe Paediatric Oncology Centres. Arch Dis Child 2001;85:12–5.
- Malviya S, Voepel-Lewis T, Tait AR. Adverse events and risk factors associated
with the sedation of children by nonanesthesiologists. Anesth Analg 1997;85:1207–13] - Cravero JP, Beach ML, Blike GT, Gallagher SM, Hertzog JH. The incidence and nature of adverse events during pediatric sedation/anesthesia with propofol for procedures outside the operating room: a report from the Pediatric Sedation Research Consortium. Anesth Analg. 2009 Mar;108(3):795-804.
- Odegard KC et al, SCAI/CCAS/SPA Expert Consensus Statement for Anesthesia and Sedation Practice: Recommendations for Patients Undergoing Diagnostic and Therapeutic Procedures in the Pediatric and Congenital Cardiac Catheterization Laboratory. Anesth Analg. 2016 Nov;123(5):1201-1209
- Faraoni, D, et al Development and Validation of a Risk Stratification Score for Children With Congenital Heart Disease Undergoing Noncardiac Surgery. Anesth & Analg. 2016 Oct. 123(4) 824–830).
- Ramamoorthy C et al. Anesthesia-related cardiac arrest in children with heart disease: data from the Pediatric Perioperative Cardiac Arrest (POCA) registry. Anesth Analg. 2010 May 1;110(5):1376-82).
- Odegard KC et al. The Frequency of Cardiac Arrests in Patients With Congenital Heart Disease Undergoing Cardiac Catheterization. Anesth Analg 118 (1), 175-182. 1 2014.
- Carmosino MJ et al, Perioperative complications in children with pulmonary hypertension undergoing noncardiac surgery or cardiac catheterization. Anesth Analg. 2007 Mar;104(3):521-7
- Taylor CJ et al. Risk of cardiac catheterization under anaesthesia in children with pulmonary hypertension. Br J Anaesth. 2007 May;98(5):657-61. Epub 2007 Mar 31.
- Chaudhry-Waterman N et al. Developing tools to measure quality in congenital catheterization and interventions: the congenital cardiac catheterization project on outcomes (C3PO). Methodist Debakey Cardiovasc J. 2014 Apr-Jun;10(2):63-7.
- Jayaram N et al. Adjusting for Risk Associated With Pediatric and Congenital Cardiac Catheterization: A Report From the NCDR IMPACT Registry. Circulation. 2015 Nov 17;132(20):1863-70.
- Nykanen EG et al. CRISP: Catheterization RISk score for Pediatrics: A Report from the Congenital Cardiac Interventional Study Consortium (CCISC). Catheter Cardiovasc Interv. 2016 Feb 1;87(2):302-9.
- Byrne ML et al. Am Heart J. 2015 Jun;169(6):823-832. Effect of center catheterization volume on risk of catastrophic adverse event after cardiac catheterization in children.





