Syndromes Review
Mucopolysaccharidoses: Clinical features and considerations for anesthetic and perioperative care
By Jared RE Hylton, MD, MS
and Dean Laochamroonvorapongse, MD, MPH
OHSU Doernbecher Children’s Hospital
Portland, Oregon
Introduction:
The mucopolysaccharidoses (MPS) are a diverse group of metabolic lysosomal storage disorders caused by the deficiency of enzymes required for the breakdown of glycosaminoglycans (GAG), also known as mucopolysaccharides. Accumulation of mucopolysaccharides in tissues leads to progressive organ dysfunction, decreased life span, and a constellation of pathologic findings. The MPS disorders are further classified into subtypes that are differentiated by specific enzyme deficiency, substrate accumulated, clinical presentation, and age of presentation.
While these disorders are relatively rare, a high percentage of these patients will present for surgery. Data from the MPS I registry (N = 544) showed that 75% of patients underwent one or more surgical procedures with a median of 3-4 surgeries per patient.(1) The Hunter Outcome Survey (N=527) showed that 83.7% of MPS II patients underwent surgical interventions.(2)
Patients with MPS disorders are at high risk for surgical and anesthetic complications. Common clinical features that can complicate otherwise routine procedures include difficult airway, impaired pulmonary function, cardiac anomalies, increased ICP, spinal cord compression, and neurologic complications. Given these perioperative risks, surgery in MPS patients is associated with a relatively high mortality rate. A study that examined a registry of MPS 1 patients that underwent a total of 4,762 procedures showed a 30-day risk of death/procedure and death/patient rates of 0.7% and 4.2%.(3)
The MPS disorders are particularly relevant to pediatric anesthesiologists given that MPS patients often present in childhood for airway procedures, cardiac surgery, and frequently for out of operating room (OOR) anesthesia for diagnostic imaging. Hurler syndrome (MPS I) has been called “the worst airway problem in pediatric anesthesia.”(4)
This review will provide an overview of pertinent clinical information for anesthesiologists caring for MPS patients.
Pathophysiology:
Patients with MPS disorders either have a quantitative or qualitative defect of one of the eleven enzymes required to break down GAG. GAG are large, unbranched polymers of repeating sulfated acidic and amino sugar disaccharide units attached to a protein core. These complex molecules are widely distributed throughout the body and serve many functions. GAG are highly polar, hydrophilic and serve as components of lubricant fluid in joints and maintain tissue hydration. GAG also serves as a surface coating that aids in binding of growth factors to cells and as building blocks for bone, cartilage, connective tissue, and a multitude of other tissues throughout the body.
GAG are recycled and replenished through metabolic stepwise degradation of the terminal sulfate, acidic, and amino sugar residues by a series of lysosomal enzymes. Quantitative or qualitative defects of any one of these enzymes impairs degradation of these substrates, accumulation of substrate, and the subsequent development of clinical phenotypes that are dependent upon the specific enzyme affected and the substrate that accumulates. In general, these disorders are autosomal recessive except for Hunter syndrome (MPS II), which is X-linked recessive.
Epidemiology:
MPS are rare disorders, but their incidence and prevalence varies widely amongst the different subtypes. For example, in the Australian population the incidence of MPS type I (Hurler-Scheie) was estimated to be 1/121,000 while the incidence of MPS type III-C (Sanfilippo C) was estimated to be 1/1.4 million.(5) The overall estimated total incidence of all subtypes aggregated together is thought to be 1/20,000 live births. (6,7)
Subtypes:
Table 1 outlines pertinent features of the main MPS subtypes.
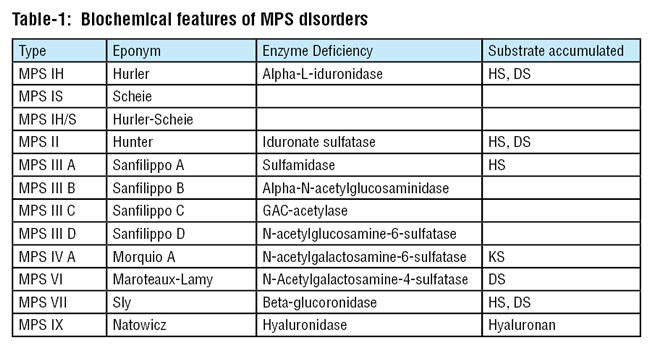
DS = Dermatan sulfate
KS = Keratin sulfate
Clinical features related to anesthetic risk:
We will highlight the main clinical findings common among the subtypes with emphasis on pathology that relates to increased risk of perioperative complications.
1.) Airway and pulmonary abnormalities:
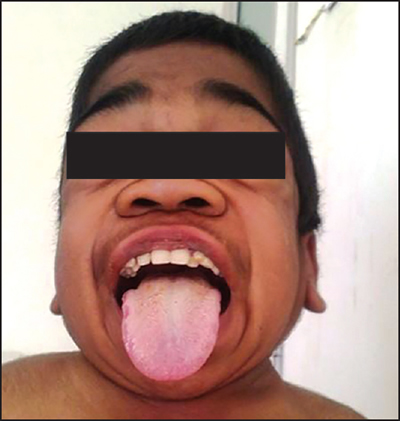
The most common serious anesthetic complications associated with MPS patients are related to difficulty with airway management. Airway obstruction is a common theme amongst all MPS subtypes. GAGs accumulate in the upper airway, which can cause airway narrowing, macroglossia, adenotonsillar hypertrophy, and thickened soft tissues in the larynx and pharynx.(8) Upper airway obstruction can be exacerbated by deformities of the skull or spine, such as mandibular abnormalities, short neck, and high anterior larynx. Abnormally thick secretions, tracheal distortion, and laxity of tracheal tissue can also compound airway obstruction.(9) These factors can lead to significant complications during sedation and general anesthesia with difficulty in mask ventilation, partial or complete airway obstruction, and post-intubation stridor and respiratory distress. A preoperative airway examination may be performed to examine for signs of obstruction using flexible nasoendoscopy with topical anesthetic. In the PACU, patients may need mask continuous positive airway pressure (CPAP) for recovery following extubation to prevent obstruction.
Many of the factors above also contribute to difficulty in direct laryngoscopy and intubation in MPS patients. GAG deposition in the larynx can impede identification of the larynx. Other features such as narrowed hypopharynx due to redundant tissue, large tongue, limited mouth opening, and atlantoaxial instability, all contribute to difficult airway management.(10) It is imperative that the anesthesiologist conduct a thorough airway examination to devise a comprehensive airway management plan. All MPS patients should be considered difficult airways and video laryngoscopy and fiberoptic scopes should be readily available. Fiberoptic intubation through a supraglottic airway device can be helpful for securing the airway of MPS patients, since the supraglottic airway device can help push away redundant tissues and assist the anesthesiologist in identifying the larynx.
Beyond difficulty with airway management, MPS patients often present with other comorbid pulmonary pathology that can complicate the perioperative period. Restrictive pulmonary disease secondary to compromised diaphragmatic excursion from enlarged liver and/or spleen as well as thoracic-cage dysfunction are common findings across many of the MPS subtypes and is not likely due to interstitial disease.(11) Progressive obstructive disease and diffusion defects have also been described. Reactive airway disease is common. Even minor upper respiratory tract infections can trigger reactive airway exacerbations and in the setting of upper airway obstruction and lung disease, these events can lead to severe respiratory compromise and even sudden respiratory arrest.(12)
2.) Spinal cord risk
Compression and compromise of the cervical spinal cord can result from either vertebral subluxation or pachymeningitis cervicalis. Atlantoaxial instability due to odontoid hypoplasia with risk of C1/C2 subluxation is common in MPS IV (Morquio syndrome), but can also occur in MPS I (Hurler-Scheie), VI, and VII.(13) For many MPS patients, extension and flexion plain films should be obtained preoperatively to assess for atlantoaxial instability. If these films are not available, it may be prudent to perform manual in-line stabilization of the head and neck during airway management to help minimize risk of C1/C2 subluxation.
Pachymeningitis cervicalis is progressive thickening and scarring of the meninges secondary to mucopolysaccharide storage.14) The thickened meninges and connective tissue can form a constrictive sleeve around the spinal cord that impairs CSF flow and can progressively compress the cervical spinal cord. Clinically this can lead to progressive motor and sensory neurologic impairment, which has been reported to impair respiratory, bowel, and bladder function. Other defects are dependent upon the extent and location of compression. This pathologic finding has been described in a number of the MPS subtypes including MPS I, II, VI, and VII.
3.) Cardiac abnormalities
Congenital cardiac lesions are common in all subtypes of MPS. Valvular disease is the most common finding as GAG deposition can lead to valvular regurgitation and/or stenosis. Mitral and aortic regurgitation are the most common lesions.(15) Severe mitral or aortic stenosis has been shown to increase mortality risk during surgery in non-MPS patients and it is reasonable to assume that this increased risk translates to MPS patients.(16)
Systolic and diastolic dysfunction have been noted in MPS patients.(10) Infants can develop fatal cardiomyopathy and acute decompensated congestive heart failure.
Early onset, diffuse coronary artery disease has also been noted as a feature associated with MPS patients.(10) Identification of MPS patients at risk for myocardial ischemia may be obscured due to patient immobility and impaired communication abilities.
As mentioned earlier, obstructive sleep apnea is a common feature across many subtypes of MPS. Untreated OSA can lead to the development of pulmonary hypertension secondary to chronic hypoxemia. While no firm guidelines exist for the preoperative evaluation for cardiac risk in MPS patients, the anesthesiologist must have a high index of suspicion for underlying cardiac pathology. Any symptoms of syncope or pre-syncope should merit a preoperative electrocardiogram (ECG) and possibly transthoracic echocardiogram (TTE). Use of invasive blood pressure monitoring may be prudent for long procedures and high risk surgery.
4.) Neurologic risks
Communicating hydrocephalus is a common feature of MPS I, II, III, VI, and VII.(17) GAGs can cause engorgement of arachnoid granulations and impede resorption of CSF, thus leading to increased intracranial pressure (ICP) .(18) Typical signs of acutely elevated ICP, such as severe headaches, visual problems, and altered mental status, may be present but the process may be insidious and the clinical signs may be difficult to interpret with underlying developmental delay. MPS patients often present for ventriculoperitoneal shunt placement and/or revisions.
Progressive developmental delay can impair cooperation and pose a challenge to the anesthesiologist during preoperative evaluation and perioperative care. It is important in the preoperative evaluation to inquire about seizure history and ensure that scheduled doses of anticonvulsants have not been missed prior to surgery. As mentioned previously, progressive spinal cord compression can lead to central sleep apnea from upper cervical cord compression. Given the risk associated with this pathologic finding as well as the risk of obstructive airway compromised discussed earlier, it is prudent to avoid or limit medications with respiratory depressant side effects, especially sedative premedication and opioids. The anesthesiologist should seek to utilize regional anesthesia, neuraxial anesthesia, and multimodal analgesia whenever appropriate.
Summary:
MPS patients have a multitude of clinical findings that place them at an increased risk for perioperative complications. Anesthetic care can be challenging and should be performed by an experienced pediatric anesthesiologist. Ideally, decisions about perioperative care should be made by a multidisciplinary team well in advance of surgery to provide ample time for coordination. With specialized expertise, the pediatric anesthesiologist plays a crucial role in helping to reduce perioperative morbidity and mortality for patients with these rare disorders.
References:
- Arn P, Wraith JE, Underhill L. Characterization of surgical procedures in patients with mucopolysaccharidosis type I: findings from the MPS I Registry. J Pediatr. 2009 Jun;154:859-864.
- Mendelsohn NJ, Harmatz P, Bodamer O, et al. Importance of surgical history in diagnosing mucopolysaccharidosis type II (Hunter syndrome): data from the Hunter Outcome Survey. Genet Med. 2010 Dec;12(12):816-22.
- Arn P, Whitley C, Wraith JE, et al. High rate of postoperative mortality in patients with mucopolysaccharidosis I: findings from the MPS I registry. J Pediatr Surg. 2012 Mar;47(3):477-84.
- Smith RM. Anesthesia for infants and children, 4th ed. St. Louis, MA: CV Mosby, 1980:533-536.
- Meikle PJ, Hopwood JJ, Claque AE, et al. Prevalence of lysosomal storage disorders. JAMA 1999 Jan 20;281(3):249-54.
- Wraith JE. The mucopolysaccharidoses: a clinical review and guide to management. Arch Dis Child 1995; 72:263.
- Muenzer J. Mucopolysaccharidoses. Adv Pediatr 1986; 33:269.
- Leighton SE, Papsin B, Vellodi A, et al. Disordered breathing during sleep in patients with mucopolysaccharidoses. Int J Pediatr Otorhinolaryngol. 2001 Apr 27;58(2):127-38.
- Pelley CJ, Kwo J, Hess DR. Tracheomalacia in an adult with respiratory failure and Morquio syndrome. Respir Care. 2007 Mar;52(3):278-82.
- Walker R, Belani KG, Braunlin EA, et al. Anaesthesia and airway management in mucopolysaccharidosis. J Inherit Metab Dis. 2013 Mar;36(2):211-9.
- Lin SP, Shih SC, Chuang CK, et al. Characterization of pulmonary function impairments in patients with mucopolysaccharidoses -- changes with age and treatment. Pediatr Pulmonol 2014;49:277.
- Semenza GL, Pyeritz RE. Respiratory complications of mucopolysaccharide storage disorders. Medicine (Baltimore) 1988; 67:209.
- Stevens JM, Kendall BE, Crockard HA, et al. The odontoid process in Morquio-Brailsford’s disease. The effects of occipitocervical fusion. J Bone Joint Surg Br 1991; 73:851.
- Wynn R. Mucopolysaccharidoses: Complications and management. In: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. (Accessed on August 20th, 2016.)
- Wippermann CF, Beck M, Schranz D, et al. Mitral and aortic regurgitation in 84 patients with mucopolysaccharidoses. Eur J Pediatr. 1995 Feb;154(2):98-101.
- Fleisher LA, Beckman JA, Brown KA, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circulation. 2009 Nov 24;120(21):e169-276.
- Sheridan M, Johnston I. Hydrocephalus and pseudotumor cerebri in the mucopolysaccharidoses. Childs Nerv Syst. 1994 Apr;10(3):148-50.
- Van Aerde J, Piets C, Van der Hauwaert L. Hydrocephalus in Hunter Syndrome. Acta Paediatr Belg. 1981 Apr-Jun;34(2):93-96.





