Wake Up Safe
Wake Up Safe Update
By Manon Haché, MD
Columbia University Medical Center
Wake Up Safe Research and Communications Officer
Don Tyler, MD
Wake Up Safe Executive Director
Wake up Safe was founded in 2008 with the goal of reducing preventable serious perioperative adverse events. We are a Patient Safety Organization, as designated by the Agency for Healthcare Research and Quality (AHRQ).
The components of the organization are threefold:
- A registry for serious adverse events among participating institutions.
- Quality improvement projects designed to reduce the occurrence of adverse events.
- Peer visits to institutions identify best practices and make recommendations about processes that could be improved.
The benefits of participation for the institutions are many. Institutions receive a report detailing their patient’s ages, types of surgery and physical status that can be compared to the average of the entire group. There is also a report of serious adverse events, which can be compared to the group. Root cause analysis for serious adverse events and recommendations for prevention are discussed at monthly teleconferences and at twice-yearly meetings. There is also opportunity to join in quality improvement projects, participate in peer evaluation site visits, as well as learn Safety Analytics and Quality Improvement methodology.
Since we last updated SPA members, Wake Up safe has grown to include 30 participating institutions. We have demographic data on 2,327,083 anesthetics, and an analysis of 2,997 serious adverse events. Over the last year, we have accomplished the following:
- Developed a curriculum for members to improve their knowledge of quality improvement work and safety analytics,
- Approved four research projects, two of which have had posters presented at national meetings,
- Reviewed all institution’s demographic data submission and audit event data submission
- Published and created courses and workshops to teach safety analytics and quality improvement science
- Continued peer visits to look for best practices and make recommendations in areas that could be improved.
We encourage participating institutions to report demographic data and serious adverse event data, employ safety analytics to identify root causes of serious adverse events, develop quality improvement projects and use high reliability organization principles to decrease serious adverse events. One of our participating institutions published their quality improvement work this year(1) describing the quality improvement methods utilized to decrease serious airway events and airway related cardiac arrests in their institution.
So far, many recommendations have come from the analysis of cases reported to Wake Up Safe.
A statement highlighting the risk of hyperkalemic cardiac arrest associated with massive transfusion in infants and children has been posted as well as a statement on preventing wrong-sided procedures emphasizing the role of anesthesiologists in the process. In addition, a warning regarding acetaminophen overdose was issued as well as a safety alert regarding the increasing frequency of IV medication errors and the need to decease this risk. These adverse events were identified because a small number of cases were noted and reported from different institutions emphasizing the importance of the problem.
Many publications have highlighted the quality improvement efforts of the group. Articles were published detailing the development of Wake Up Safe as a National Pediatric Anesthesia Quality Improvement program (2) and explaining the application of root cause analysis in our organization as a quality improvement measure.(3) Other articles used root cause analysis to describe specific cases such as bupivacaine overdose leading to ventricular tachycardia (4) and wrong site surgery. (5) After multiple cases were reported to Wake Up Safe, an examination of the existing literature was performed and recommendations were made concerning the prevention of hyperkalemia leading to cardiac arrest in pediatric patients receiving massive transfusion.(5)
Our database has now surpassed two million reported cases. The incidence of reported serious adverse events is 8.8 per 10,000 cases. The types of serious adverse events reported are illustrated below. Anesthesia was identified as the primary cause of the event is 0.004 per 10,000 cases, and 0.1 per 10,000 cases for anesthesia as the secondary cause for the event.
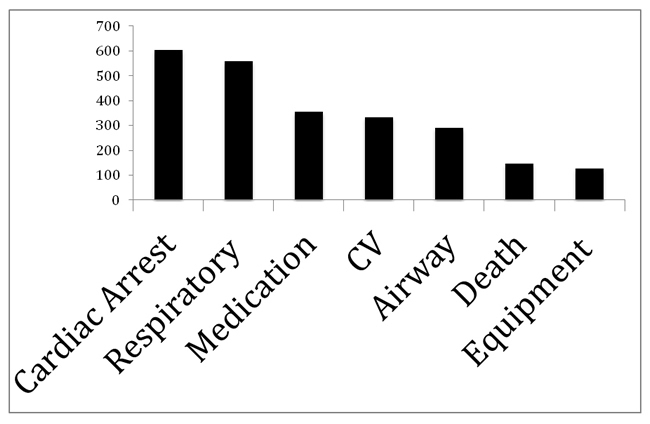
CV: Cardiovascular support
Peer visits have identified a number of best practices that we recommend institutions adopt, including:
CULTURE OF SAFETY
- A robust system for self
- reporting of adverse events by anesthesiologists.
- Departmental peer review and safety combined in the same committee
- Use of EMR to find patients who visit the ER within 24 hours of anesthesia
- Preoperative check of all existing IVs
- Separation of clean and dirty areas in the anesthesia work space
UNIVERSAL PROTOCOL
- A morning meeting or “huddle” that includes anesthesiology, surgery, and nursing to discuss cases and operational issues for the day.
- Sign in script in electronic record with a picture of patient to aid identification
- Perform sign-in with parents present
- Time out done with challenge-response format
- Consistent time out prior to anesthesia blocks
HANDOFFS
- Pre-anesthesia clinic staffed by an attending anesthesiologist
- Preoperative report generated from EMR including important information for anesthesiologist
- Use of scripts during handoffs, having PACU nurse fill out the form.
- Attending to attending communication during handoff of ICU patients with a written summary
MEDICATION MANAGEMENT
- If locked medication carts are used, have a mechanism to enter cart in an emergency
- Use of APSF recommendations as a framework for improving medication safety
THE FOLLOWING ARE ADOPTED FROM THE APSF RECOMMENDATIONS FOR MEDICATION SAFETY
- High alert drugs (such as phenylephrine and epinephrine) should be available in standardized concentrations/diluents prepared by pharmacy in a ready-to-use (bolus or infusion) form that is appropriate for both adult and pediatric patients.
- Infusions should be delivered by an electronically-controlled smart device containing a drug library.
- Ready-to-use syringes and infusions should have standardized fully compliant machine–readable labels.
- Interdisciplinary and uniform curriculum for medication administration safety to be available to all training programs and facilities.
- No concentrated versions of any potentially lethal agents in the operating room.
Wake up Safe will continue to encourage reporting of events and to teach safety analytics and quality improvement through coursework, workshops, and site visits at member institutions.
References
- Spaeth JP, Kreeger R, Varughese AM, Wittkugel E. Interventions designed using quality improvement methods reduce the incidence of serious airway events and airway cardiac arrests during pediatric anesthesia. Paediatric Anaesthesia. 2016 Feb;26(2):164• 72. PubMed PMID: 26693705.
- Kurth CD, Tyler D, Heitmiller E, Tosone SR, Martin L, Deshpande JK. National pediatric anesthesia safety quality improvement program in the United States. Anesthesia and Analgesia. 2014 Jul;119(1):112• 21. PubMed PMID: 24413551.
- Tjia I, Rampersad S, Varughese A, Heitmiller E, Tyler DC, Lee AC, et al. Wake Up Safe and root cause analysis: quality improvement in pediatric anesthesia. Anesthesia and Analgesia. 2014 Jul;119(1):122• 36. PubMed PMID: 24945124.
- Buck D, Kreeger R, Spaeth J. Case discussion and root cause analysis: bupivacaine overdose in an infant leading to ventricular tachycardia. Anesthesia and Analgesia. 2014 Jul;119(1):137• 40. PubMed PMID: 24945125.
- Rampersad S, Rossi MG, Yarnell C, Uejima T. Wrong site frenulectomy in a child: a serious safety event. Anesthesia and Analgesia. 2014 Jul;119(1):141• 4. PubMed PMID: 24945126.





