PRO/CON
PRO
Getting with the Times: Cuffed Endotracheal Tubes are Safe in Pediatric Patients
 By Surendrasingh Chhabada, MD; Lauren K. Licina, MS, MD; and Dorothea A. Markakis, MD, FAAP
By Surendrasingh Chhabada, MD; Lauren K. Licina, MS, MD; and Dorothea A. Markakis, MD, FAAP
Cleveland Clinic Department of Pediatric Anesthesia
Cleveland, Ohio
Conventional teaching for pediatric anesthesiologists and intensivists has been that uncuffed endotracheal tubes (ETT) are the standard of care in patients under the age of 8 years. The reasoning behind this is based upon century old cadaveric studies of infant and children’s airway specimens (1, 2). The pediatric larynx was thought to be funnel shaped with the narrowest part being the non-distensible cricoid cartilage which was circular (3). Based on these anatomical studies, the use of an uncuffed ETT was thought to reduce the risk of complications like post-extubation stridor, pressure related tracheal injuries and subglottic stenosis. We present scientific evidence to support the soundness of this reasoning. The introduction of polyvinyl chloride (PVC) and high volume low pressure (HVLP) cuffed ETTs may strengthen the argument for routine use of cuffed ETTs. We review the literature and reach a conclusion which will allow us to provide the most safe and effective care for our patients.
Recent research on the pediatric airway utilizing video bronchoscopy and radiological imaging in spontaneously breathing, sedated and paralyzed children has improved our understanding of the pediatric airway (4, 5, 6). These more recent studies conclude that the pediatric airway is more cylindrical, like an adult's airway, rather than funnel shaped. The cricoid is not round, but rather elliptical in shape with the transverse diameter being less than anteroposterior diameter and the narrowest part of larynx is the rima glottis/subglottic region.
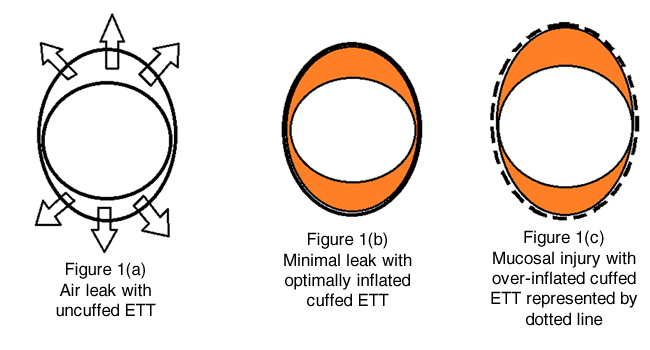
Use of circular uncuffed ETTs will invariably cause air leak antero-posteriorly in an elliptical shaped airway (figure 1A). An appropriately sized uncuffed tube can cause less air leak but wedges along the cricoid ring and can potentially damage the lateral walls. In contrast, a cuffed ETT will come in contact with the trachea and U shaped cartilages creating a seal with the cuff. Those U-shaped cartilages have a muscular posterior wall providing more compliance than the cricoid ring. The ideal endotracheal tube should be large enough to seal the cricoid ring, yet small enough to allow an air leak at peak inspiratory pressures between 20-25 cm H2O as in figure 1B (orange represents the inflated cuff). This will allow adequate positive pressure ventilation without exerting excessive pressure on the tracheal mucosa thus decreasing the risk of pressure related injuries. An endotracheal tube that is too large or an over-inflated cuff can cause local ischemia, deep ulceration, secondary submucosal collagen formation and scar tissue, resulting in potentially permanent tracheal damage as shown in figure 1C.
With the development of HVLP cuffed ETTs and ultrathin polyurethane Microcuff pediatric ETTs, there has been a change in clinical practice with a preference for the use of cuffed ETTs in management of the pediatric airway. HVLP cuffs are the standard of care in adults. Microcuff tubes have HVLP cuffs made from an ultra-thin polyurethane cuff (10 μm), which does not form folds and channels between the cuff and the tracheal wall. There is no Murphy eye, so the cuff is more distal on the ETT shaft. The cuff is short and when inflated, it expands below the sub-glottis, providing a seal with cuff pressure less than 10 cm H2O. It has correctly placed depth markings; not surprisingly, it has a low rate of tube exchange (7,8,9). These new tubes are thought to improve sealing and avoid mucosal irritation.
Predicting an appropriate size for an uncuffed ETT can be difficult and sizing formulas are often inaccurate. Placement of an undersized tube will create a large air leak resulting in poor minute ventilation, difficult to maintain positive end expiratory pressure (PEEP) and worsening atelectasis. Undersized tubes can lead to inaccurate respiratory monitoring, inaccurate assessment of inhaled anesthetic agents, and increased risk of aspiration. This might have practical implications in clinical practice for cases with varying pulmonary compliance intraoperatively or postoperatively, mandating higher driving pressures to provide optimal oxygenation and ventilation to the patient. Examples include diaphragmatic hernia repair, omphalocele or gastroschisis repair, thoracic surgeries, and laparoscopic surgeries. Mahmoud et al. (10) showed that 42.3% of all neonates studied with an uncuffed ETT experienced a leak of >40% at some point. Additionally, Dorsey et al. (11) found a clinically significant ETT leak in 19.4% of infants (0–2 years) with burns who had uncuffed ETTs in their study.
If the air leak around the uncuffed ETT is too large, additional laryngoscopy and intubation attempts for ETT exchange may be required, with the potential for damage to the airway. Khine et al. conducted a quasi-randomized controlled trial (RCT) and showed reintubation rates of 0% with cuffed ETT use as compared with 30% with uncuffed ETT use in 488 young children (12). A multi-centric prospective randomized controlled trial by Weiss et al showed reintubation rates of 2.1% with cuffed ETTs and 30.8% with uncuffed ETTs in 2246 children ages ranged from birth to 5 years, p<0.0001 (13). Dullenkopf et al. (14) showed that the tube exchange rate with cuffed ETTs was only 1.6% in 500 children ages ranged from birth to 16 years. This data confirms that intubation with cuffed ETTs results in fewer intubations, and by extension, fewer potential complications from repeated laryngoscopy.
ETT cuff leak varies with duration of intubation, changes in head position, type of anesthetic gas, level of sedation and neuromuscular blockade as well as changes in pulmonary compliance (15, 16, 17). Thus, a tube might be appropriate when initially placed, but an alteration of any of the above factors could create an air leak requiring tube exchange. Tube exchange can be extremely challenging in the difficult airway scenario and puts patients at risk for intubation related injury. Cuffed ETTs offer flexibility by providing the option to adjust cuff volume and decrease air leak without requiring reintubation of the patient.
Additional benefits of the seal obtained with cuffed ETTs include safety and precision in control of ventilation. Effective sealing of the airway is extremely important in oropharyngeal surgeries such as adenotonsillectomy where there is an increased risk of airway fire (18, 19). The Practice Advisory for the Prevention and Management of Operating Room Fires from the American Society of Anesthesiologists states: “For cases involving surgery inside the airway, consultants and ASA members both agree that a cuffed endotracheal tube should be used instead of an uncuffed endotracheal tube when medically appropriate” (20). Effective sealing also ensures proper ventilation in maintaining partial pressure of CO2 in blood. This can be crucial in patients with elevated intracranial pressure and pulmonary hypertension. Hence, in these critically ill patients, a cuffed ETT will provide safer and more controlled ventilatory management.
An air leak around an uncuffed ETT can lead to increases in unscavenged waste and subsequent unnecessary exposure of operating room (OR) personnel to that waste. Fresh gas flows may need to be increased to compensate for a large air leak, which poses economic implications along with loss of heat and humidity. Although cuffed ETTs are more expensive, decreased leak can reduce unscavenged waste, resulting in less fresh gas flow and a more efficient anesthetic. Eschertzhuber et al reported in 70 patients that with the use of cuffed ETTs decreased the costs by 19 EUR/patient (21). Murat et al. (22) reported a dramatic decrease in the concentration of sevoflurane and nitrous oxide with use of cuffed endotracheal tube in pediatric operating rooms.
Gopalareddy et al studied micro-aspiration in 27 patients aged 4 months to 19 years who required mechanical ventilation with cuffed and uncuffed ETTs in the pediatric intensive care unit (PICU). Micro-aspiration was determined by measuring gastric pepsin in tracheal aspirates. The authors reported 100% micro-aspiration in the uncuffed ETT group compared to 53% in the cuffed tube group (23). Browning et al reported similar results with a significant decrease in the incidence of aspiration determined by dye positive tracheal aspirates in the cuffed ETT group (24). In emergency and laparoscopic surgeries, there is an inherent increased risk of aspiration. The use of cuffed ETTs in these cases provides the benefit of an effective seal.
There are several prospective studies examining the incidence of airway complications when using cuffed versus uncuffed ETTs. Shi et al. conducted a systematic review and meta-analysis in 2014 including two RCTs and two prospective cohort studies comparing the use of cuffed and uncuffed ETTs in children. The authors found that use of cuffed ETTs does not increase the incidence of post-extubation stridor and the tube exchange rate was lower in cuffed ETT group (25). Wit et al. recently published a retrospective study reviewing postoperative respiratory complications in children between the ages of 0 and 7 undergoing anesthesia and requiring intubation. The primary outcome examined the incidence of postoperative stridor, wheezing, dyspnea and any interventions needed in the post anesthesia care unit (PACU). They found that cuffed tubes were used in 77% of all cases examined and in 61% of cases involving infants. When examining the entire patient cohort, there was no difference in postoperative respiratory complications between the patients with cuffed tubes and uncuffed tubes. Interestingly, they also showed an increase in the use of cuffed tubes in more recent years, in both infants and children (26). While this information is reassuring for the safety of cuffed endotracheal tubes, some criticize that the lack of post-extubation stridor is not an adequate enough evaluation for lack of harm caused by tracheal tubes as subglottic stenosis (SGS) may manifest later. Manica et al. addressed this issue by performing flexible fiberoptic laryngoscopy on every intubated patient in the intensive care unit to diagnose SGS and other laryngeal lesions by direct visualization. The patients studied were between the ages of 0 and 5 who had been intubated for longer than 24 hours. They found no increased risk of SGS or laryngeal lesions with the use of cuffed ETT in pediatric ICU patients (27).
Children with congenital heart disease undergoing repair on cardiopulmonary bypass represent another group of patients that have been studied for airway complications. As a result of their surgical procedure and inherent pathophysiology, these patients often leave the operating room edematous, potentially in a low cardiac output state, and on vasoconstrictor medications. These factors all impair transcapillary perfusion to the laryngeal mucosa. Patients with cyanotic lesions are at even higher risk for airway ischemia. Mossad et al retrospectively examined all children with congenital heart disease undergoing surgical repair over a four year period. They showed a decreased rate of SGS in the cuffed ETT group versus prior studies in which uncuffed tubes were predominantly used. Additionally, 15 out of 17 children who developed SGS were intubated with uncuffed ETT. All children with SGS were under two years of age. Further analysis revealed that children less than one year of age were at higher risk of developing SGS compared to children aged 1 to 2 years (28). Kruse et al also showed via a retrospective study that the presence of a cuffed ETT was not associated with the development of SGS in the congenital cardiac population undergoing CPB surgery. They also found that the mean age for developing SGS was 4.5 months and a higher incidence of SGS in infants less than 1 month old, again supporting the notion that infants are at the highest risk of developing airway complications from ETT (29).
The more recent advent of the Microcuff ETT in 2004 addresses some issues experienced with the HVLP ETTs, as discussed above. Of note, the manufacturer of Microcuff ETT does not recommend its use in infants less than 3 kg. Thomas et al. recently performed a small retrospective cohort study comparing the use of Microcuff tubes versus uncuffed ETTs in infants of 2 to 3 kg in a single-center tertiary care NICU. All 23 patients were intubated with a Microcuff tube for surgical procedures by anesthesiologists in the OR. Infants who received an uncuffed ETT served as controls. The author found no significant difference in rates of ETT blockage, post extubation stridor, post extubation use of dexamethasone or epinephrine or re-intubation for stridor. They also did not find any difference in rates of atelectasis and pneumonia. Hence, in this small study there was no difference in rates of airway complications with the use of Microcuff ETTs in neonates less than 3 kg (30). Weiss et al. in 2009 also showed that use of Microcuff ETT in the 0 to 5 year age group was associated with decreased reintubation rate and no change in post extubation stridor when compared with uncuffed ETTs (13). Additional study is needed to establish the safety of Microcuff tubes in small neonates.
As pediatric anesthesiologists, we care for the smallest, most vulnerable patients. It is our responsibility and duty to keep our patients safe from iatrogenic and other harm. The benefits of cuffed ETTs include lower reintubation rate, less chance of air leak, improved ventilation, tighter and reliable control of carbon dioxide, maintenance of PEEP, less waste of volatile agents, less risk of aspiration and overall better airway control for select surgical procedures. Recent research in the form of retrospective and prospective trials fails to show that cuffed ETTs lead to negative airway complications (12, 13, 22, 22, 24, 25, 26, 27). Admittedly, there are theoretical disadvantages of cuffed ETTs including the smaller diameter with ensuing difficulties with suctioning of secretions, increased ETT resistance with increased ventilator requirements, limited use in smaller patients and potential injury from over-inflated cuffs. Further research is needed to identify the best ETT choice for the neonates, specifically those less than 3 kg. Regardless of ETT selection, as experts in airway management, we must fully understand the benefits and risks associated with the tools at our disposal. Ensuring an adequate leak around all ETTs is of paramount importance. Physiologic changes occurring during surgery and the perioperative period may alter that leak regardless of our selection of ETT. With constant vigilance, careful selection of ETT type, size and placement, periodic monitoring of ETT cuff pressure, and early extubation, we can avoid inadvertent injury to our patients while providing the benefits of intubation with a cuffed ETT.
References
- Bayeux. Tubage de larynx dans le croup. Presse Med 1897; 20: 1.
- Eckenhoff J. Some anatomic considerations of the infant larynx influencing endotracheal anesthesia. Anesthesiology 1951; 12 (4): 401-410.
- Uejima T. Cuffed endotracheal tubes in pediatric patients. Anesthesia & Analgesia 1989; 68:423.
- Wani TM. Bissonnette B, Rafiq Malik M, et al. Age-based analysis of pediatric airway dimensions using computed tomography imaging. Paediatric Pulmonology 2015; 51 (3) 267-71.
- Litman RS, Weissend EE, Shibata D, et al. Developmental changes of laryngeal dimensions in unparalyzed, sedated children. Anesthesiology 2003; 98: 41–50.
- Dalal PG, Murray D, Messner AH, et al. Pediatric laryngeal dimensions: an age-based analysis. Anesth Analg 2009;108:1475–9.
- Weiss M, Gerber AC, Dullenkopf A. Appropriate placement of intubation depth marks in a new cuffed paediatric tracheal tube. British Journal of Anaesthesia 2005; 94 :80-7.
- Weiss M, Balmer C, Dullenkopf A, Knirsch W, Gerber AC, Bauersfeld U, Berger F. Insertion depth markings allow an improved positioning of endotracheal tubes in children. Canadian Journal of Anaestheia 2005; 52 (7) 721-6.
- Weiss M, Dullenkopf A, Bottcher S, Schmitz A, Stutz K, Gysin C, Gerber AC. Clinical evaluation of cuff and tube tip position in a newly designed paediatric preformed oral cuffed tracheal tube. British Journal of Anaesthesia 2006; 97: 695-700.
- Mahmoud RA, Proquitté H, Fawzy N, et al. Tracheal tube air leak in clinical practice and impact on tidal volume measurement in ventilated neonates. Pediatric Critical Care Medicine 2011;12:197–202.
- Dorsey DP, Bowman SM, Klein MB, et al. Perioperative use of cuffed endotracheal tubes is advantageous in young pediatric burn patients. Burns 2010;36:856–60.
- Khine HH, Corddry DH, Kettrick RG, et al. Comparison of cuffed and uncuffed endotracheal tubes in young children during general anesthesia. Anesthesiology 1997; 86: 627–31.
- Weiss M, Dullenkopf A, Fischer JE, et al. Prospective randomized controlled multi-centre trial of cuffed or uncuffed endotracheal tubes in small children. British Journal of Anaesthesia 2009;103:867–73.
- Dullenkopf A, Gerber AC, Weiss M. Fit and seal characteristics of a new paediatric tracheal tube with high volume-low pressure polyurethane cuff. Acta Anaesthesiol Scand 2005; 49: 232–7.
- Main E, Castle R, Stocks J, et al. The influence of endotracheal tube leak on the assessment of respiratory function in ventilated children. Intensive Care Medicine 2001;27:1788–97.
- Finholt DA, Henry DB, Raphaely RC. Factors affecting leak around tracheal tubes in children. Canadian Anaesthetists’ Society Journal 1985; 32: 326–9.
- Suominen P, Taivainen T, Tuominen N, et al. Optimally fitted tracheal tubes decrease the probability of post extubation adverse events in children undergoing general anesthesia. Paediatric Anaesthesia. 2006;16 (6): 641-647.
- Mattucci KF, Militana CJ. The prevention of fire during oropharyngeal electrosurgery. Ear Nose Throat J 2003; 82: 107–109.
- Kaddoum RN, Chidiac EJ, Zestos MM et al. Electrocautery-induced fire during adenotonsillectomy: report of two cases. J Clin Anesth 2006; 18: 129–131.
- Apfelbaum JL, Caplan RA, Barker SJ et al. Practice advisory for the prevention and management of operating room fires: an updated report by the American Society of Anesthesiologists Task Force on Operating Room Fires. Anesthesiology 2013; 118: 271–290.
- Eschertzhuber S,Salgo B,Schmitz A,Roth W,Frotzler A,Keller CH,et al.Cuffed endotracheal tubes in children reduce sevoflurane and medical gas consumption and related costs. Acta Anaesthesiol Scand.2010;54:855-8
- Murat I. Cuffed tubes in children: a 3-year experience in a single institution. Pediatric Anaesth 2001;11:748-9.
- Gopalareddy V, He Z, Soundar S, Bolling L, Acta Paediatr. 2008 Jan; 97(1): 55–60.
- Browning DH, Graves SA. Incidence of aspiration with endotracheal tubes in children. Journal of Pediatrics 1983; 102: 582-584.
- Shi F, Xiao Y, Xiong W, Zhou Qin, Huang X: Cuffed versus uncuffed endotracheal tubes in children: a meta-analysis. Journal of Anesthesia 2016; 30: 3-11.
- De Wit M, Peelen LM, et al. The incidence of postoperative respiratory complications: A retrospective analysis of cuffed vs uncuffed tracheal tubes in children 0-7 years of age. Paediatr Anaesth. 2018;28(3):210-217.
- Manica D, Schweiger C, Caudura Mrostica PJ, Kuhl Gabriel, Antonacci Carvalho PR: Association between length of intubation and subglottic stenosis in children. The Laryngoscope 2013; 123: 1049-1054.
- Mossad E, Youssef G. Subglottic stenosis in children undergoing repair of congenital heart defects. Journal of Cardiothoracic and Vascular Anesthesia 2009; 23 (5): 658-662.
- Kruse K, Purohit P, Cadman C, Su F, Aghaeepour N, Hammer G. Subglottic stenosis following cardiac surgery with cardiopulmonary bypass in infants and children. Pediatric Critical Care Medicine. 2017; 18 (5): 429-433.
- Thomas RE, Shripada R, Minutillo C, Hullett B, Bulsara M. Cuffed endotracheal tubes in infants less than 3 kg: A retrospective cohort study. Pediatric Anesthesia. 2018;28:204-209.






