wake up safe update
By Manon Haché, MD
Columbia University Medical Center
Wake Up Safe Research and Communications Officer
Contributors
Dean Kurth MD (Cincinnati Children’s Medical Center), Wake Up Safe president; Don Tyler MD (Children’s Hospital of Philadelphia), Wake Up Safe executive director; Angela Lee MD (Children’s National Medical Center); Amanda Lorinc MD (Vanderbilt University Medical Center); Lena S. Sun MD (Columbia University Medical Center); Wake Up Safe research & communications committee members
Wake up Safe was founded in 2008 with the goal of reducing preventable serious perioperative adverse events. We are a Patient Safety Organization, as designated by the Agency for Healthcare Research and Quality (AHRQ).
Serious perioperative adverse events, and those specifically related to pediatric anesthesia, are rare. Since any single institution would have very few events, an organization like Wake Up Safe that includes participation from many institutions provides the platform for more systematic study. It is with the commitment of many individuals that Wake Up Safe has grown over the past 7 years to include 29 participating institutions.
We have been able to collect demographic data describing our patients and an analysis of serious adverse events. The benefits of participation for the institutions are many. Institutions receive a report detailing the patient’s ages, types of surgery and physical status that can be compared to the average of the entire group. There is also a report of the serious adverse events, which can be compared to the group. Root cause analysis for serious adverse events and recommendations for prevention are discussed at monthly teleconferences and at twice-yearly meetings. There is also the opportunity to join in quality improvement projects, participate in peer evaluation site visits, as well as learn Safety Analytics and Quality Improvement methodology.
Some of our accomplishments include:
- Development of specific definitions of serious adverse events,
- Implementation of a system to report serious adverse events,
- Institution of a peer visitation program to identify best practices amongst member institutions, and
- Publication and creation of courses and workshops to teach safety analytics and quality improvement science.
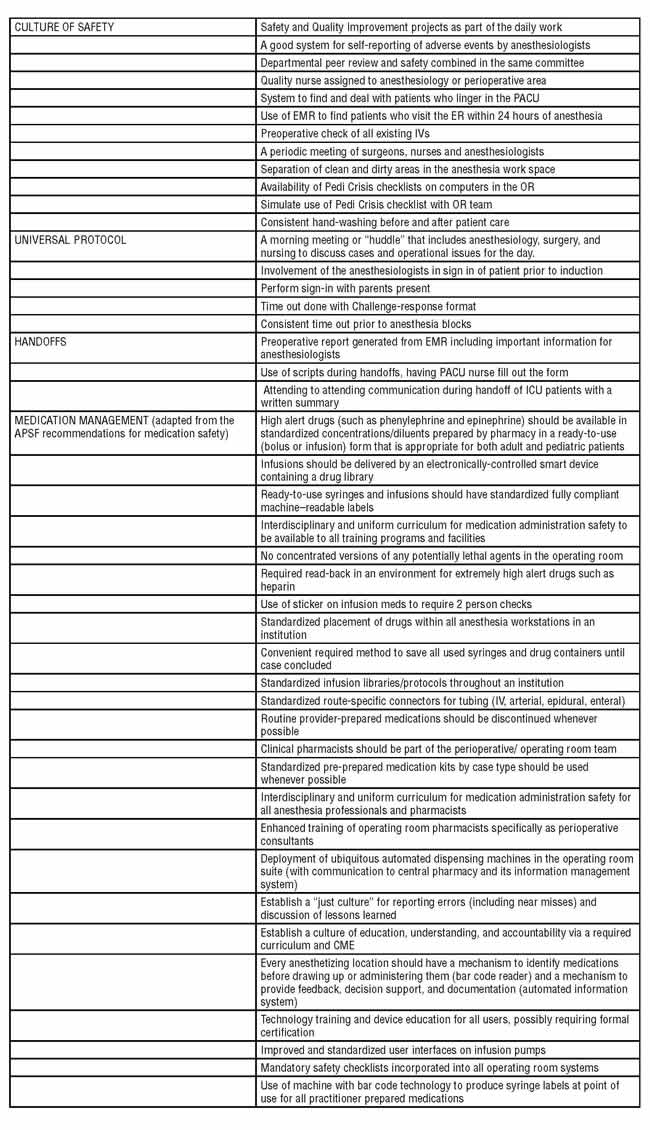
Through the peer visits, and from recommendations of the APSF, we have developed a list of safe medication practices. Other projects member institutions have undertaken include the following:
We encourage participating institutions to report demographic data and serious adverse event data, employ safety analytics to identify root causes of serious adverse events, and to develop quality improvement projects and use high reliability organization principles to decrease serious adverse events.
So far, many recommendations have stemmed from the work of Wake Up Safe. A statement highlighting the risk of hyperkalemic cardiac arrest associated with massive transfusion in infants and children has been published, a statement on preventing wrong-sided procedures has been posted emphasizing our role in the process, and a warning regarding acetaminophen overdose has been posted. These adverse events were identified because a small number of cases were noted and reported from different institutions emphasizing the importance of the problem. Recently, a safety alert was released/announced regarding the increasing frequency of IV medication errors and the need to decease this risk.
Many publications have highlighted the quality improvement efforts of the group. Articles were published detailing the development of Wake Up Safe as a National Pediatric Anesthesia Quality Improvement program (1) and explaining the application of root cause analysis in our organization as a quality improvement measure (2). Other articles used root cause analysis to describe specific cases such as bupivacaine overdose leading to ventricular tachycardia (3) and wrong site surgery (4). After multiple cases were reported to Wake Up Safe, an examination of the existing literature was performed and recommendations were made concerning the prevention of hyperkalemia leading to cardiac arrest in pediatric patients receiving massive transfusion.(5)
Our database has now surpassed one million reported cases. The incidence of reported serious adverse events with anesthesia as the primary cause of the event is 0.17 per thousand cases, and 0.33 per thousand cases for anesthesia as the secondary cause for the event. We will continue to encourage reporting of events and to teach safety analytics and quality improvement through coursework, workshops, and site visits at member institutions. Recently, we have developed an application process for access to Wake Up Safe data to promote research using our database. The results of this research will continue to inform us on our outcomes and help guide quality improvement efforts. Our strategy to improve safety is creating high reliability organizations, specifically anesthesia departments and perioperative services, by embedding safety analytics and quality improvement science into each member institution.
References
- Kurth CD, Tyler D, Heitmiller E, Tosone SR, Martin L, Deshpande JK. National Pediatric Anesthesia Safety Quality Improvement Program in the United States. Anesth Analg 2014;119:112.21
- Tjia I, Rampersad S, Varughese A, Heitmiller E, Tyler DC, Lee, AC, Hastings LA, Uejima T. Wake Up Safe and Root Cause Analysis: Quality Improvement in Pediatric Anesthesia. Anesth Analg 2014;119:122.36)
- Buck D, Kreeger R, Spaeth J. Case Discussion and Root Cause Analysis: Bupivacaine Overdose in an Infant Leading to Ventricular Tachycardia. Anesth Analg 2014;119: 137-40.
- Rampersad S, Rossi M, Yarnell C, Uejima T. Wrong Site Frenulectomy in a Child: A Serious Safety Event. Anesth Analg 2014;119:141-4.
- Lee AC, Reduque LL, Luban NL, Ness PM, Anton B, Heitmiller ES. Transfusion-associated hyperkalemic cardiac arrest in pediatric patients receiving massive transfusion. Transfusion. 2014: 54:244-54.