Pediatric Anesthesiology 2018 Reviews
Friday Session V:
Cardiac Refresher: The Broken Heart - Clinical Assessment and Management of the Pediatric Patient with Heart Disease
Reviewed by Nicole Dobija, MD
C.S. Mott Children’s Hospital
University of Michigan
The last session on Friday was a cardiac refresher: The Broken Heart - Clinical Assessment and Management of the Pediatric Patient with Heart Disease. It was moderated by James P. Spaeth, MD (Cincinnati Children’s Hospital) and encompassed four separate lectures on tools for preoperative screening.
The first lecture was “Tools for Preoperative Screening of the Pediatric Patient with Heart Disease,” from Joanna Rosing Paquin, MD (Cincinnati Children’s Hospital). Dr. Paquin provided a wonderful synopsis of how to organize the preoperative assessment for pediatric patients with heart disease. Since congenital heart disease is the most common of all congenital anomalies and there is improved long term survival for these patients, we, as anesthesia providers, will encounter more of these patients for procedures and surgeries. The POCA registry has shown that there is increased morbidity and mortality perioperatively, for patients with cardiomyopathy, single ventricle, pulmonary hypertension and left ventricle outflow tract obstruction.
Dr. Paquin began with an evaluation of known heart disease, which included a thorough history and physical. In the history it is important to know the anatomy of the cardiac lesion, the current state of the cardiopulmonary condition, recent or recurrent infections, multisystem comorbidities, previous surgeries, procedures and anesthetics, recent imaging studies and cardiology evaluation and medications and allergies.
Dr. Paquin divided the history down farther to focus on some key areas, the first being cardiopulmonary reserve. There should be a good evaluation of the patient’s activity tolerance and changes from general health. Heart failure will present differently for children at different ages. The neonate and infant may have feeding difficulties, tachypnea, diaphoresis and failure to thrive. Children may have a decreased activity level and inability to keep up with their peers. Older children may complain of fatigue, dyspnea and chest pain or have palpitations or syncope. If the child complains of palpitations or experiences arrhythmias, it is important to determine what type of rhythm disturbance has occurred and the associate symptoms and how the arrhythmia is terminated.
She went on to further discuss left ventricle outflow tract obstruction (LVOTO) signs and symptoms such as syncope (or pre-syncope), angina, CHF, systolic ejection murmur, and cardiac artery thrill. There is a risk of pulmonary hypertension and coronary ischemia that increases with the severity of LVOTO. Patients who present with pulmonary hypertension may have a reduced exercise capacity, fatigue, cough, cyanosis and abdominal pain. Imaging studies should include an echocardiogram and possible cardiac catheterization to access pulmonary vascular reactivity and pulmonary to systemic pressure ratio. Their medications may include oxygen, inhaled nitric oxide or another pulmonary vasodilator such as sildenafil or remodulin. The patient who present with current cyanosis presents its own challenges. The underlying cause of the cyanosis must be ascertained along with what (if anything) makes the cyanosis better or worse. Baseline saturations should be noted prior to any oxygen therapy.
For the middle part of the lecture, Dr. Paquin discussed the physical exam features of the patient with known congenital heart disease. On observation, are they active or lethargic, cyanotic, in any signs of distress and then look at their vital signs and where (if) they deviate from normal. On physical exam, a good airway exam is a must. Try to determine if there is respiratory versus cardiac pathology on auscultation, does the patient have wheezing or rales. Look for signs of heart failure such as jugular venous distention, hepatomegaly, and look at the extremities for tissue perfusion, edema, clubbing and for how difficult vascular access may be. When listening for heart sounds and hearing a new murmur, you must decide if it is innocent or pathologic. Innocent murmurs are low intensity and low frequency. The sound increases in intensity when supine, with increased cardiac output and decrease when upright. Pathologic murmurs are caused by an underlying structural abnormality. There may be an associated click or precordial heave present.
Dr. Paquin then presented three cases to illustrate her presentation. The first patient is a 7-year-old boy for an upper endoscopy at an outpatient center. He has a 3-week history vomiting, weight loss, anorexia and difficulty walking up the stairs to his bedroom. His CBC and electrolyte panel are within normal limits. His family tells you of a recent viral illness that preceded the symptoms he is now experiencing. After thorough history and physical you are concerned for viral cardiomyopathy.
The second case is a 15-year-old girl for tonsillectomy for chronic tonsillitis. She reports that she occasionally feels that her heart is racing and she becomes light-headed and needs to lie down. She is otherwise very active and plays tennis. Could she have SVT?
The third case is a 12-year-old boy scheduled for an open reduction internal fixation of a forearm fracture. He is a football player and reports an episode of syncope at football practice this summer which was attributed to dehydration. On examination you hear a systolic murmur. Could there be concern for LVOTO?
What to do with the information you have now collected. You must understand the planned procedure and the utility of multidisciplinary discussions. After the detailed history and physical you must ask yourself is the patient optimized for the procedure? If not cardiology consultation may be warranted. Cardiology consultation may be requested when there is a new finding of murmur, cyanosis, decreased exercise tolerance, sweating, poor weight gain, chest pain or syncope, arrhythmia, precordial heave or any associated syndromes. Even a quick telephone call to cardiology is helpful if your patient has any concerning signs or symptoms.
The second lecture was “Tools for Preoperative Screening: The Pediatric ECG” presented by Jennifer L. Hernandez, MD (University of Texas, Southwestern). The goals of the lecture stated by Dr. Hernandez were to discuss a systematic approach for the evaluation of pediatric ECGs, examine common pediatric ECG patterns, and review normal variants and age-related findings. Dr. Hernandez showed a systematic approach to EKG interpretation, that starts with the rate, rhythm and axis. Count the tick marks on the EKG, the time from tick mark to tick mark is six seconds (on the upper portion of the EKG strip). Make sure that the rate is appropriate for the child’s age based on age variations. Next is to make sure the patient has a normal rhythm and p wave morphology and there are no rhythm disturbances noted.
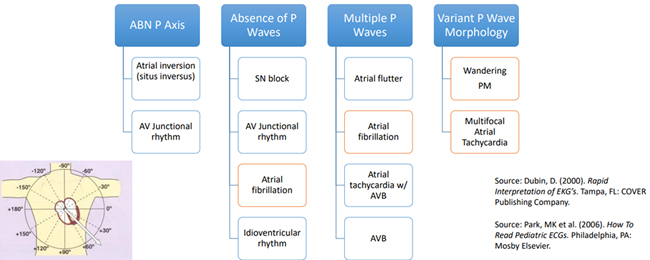
After determining a normal rhythm and p wave, the axis of the EKG should be determined. The axis of the EKG pattern varies with age. Newborns have a QRS axis of 110o, and as the patient grows and reaches adulthood the axis shifts to approximately 50o.
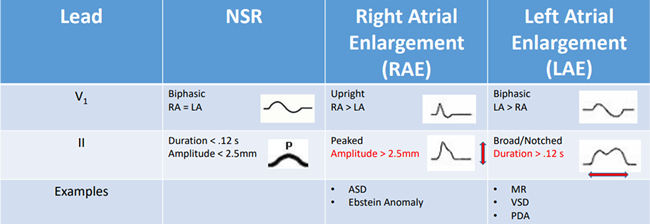
Step 2 in the systematic approach described by Dr. Hernandez is to evaluate the AV conduction by looking at the PR interval and the P wave. A normal PR interval is < 0.2 seconds. A short PR interval may be indicative or Wolf Parkinson White (WPW), Lown-Ganong-Levine, or cardiomyopathies such as Pompe’s disease. A prolonged PR interval may be seen with first degree AV block, myocarditis, toxicities such as quinidine, hypoxemia or ischemia, hyperkalemia or congenital heart disease. Variable PR intervals are associated with a wandering atrial PM and Wenckebach. There are age and heart rate related changes for PR interval that must be considered. The chart below shows different types of p wave morphology and those related abnormalities.
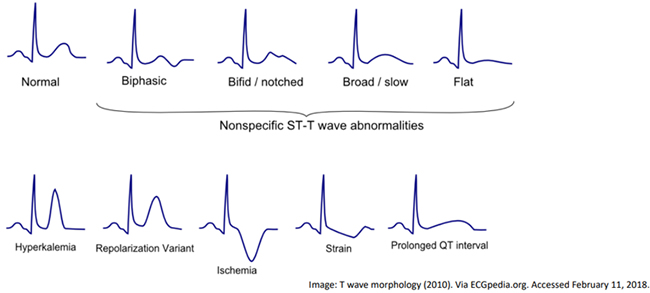
Step three in this approach is the QRS complement and to evaluate the QRS complex, ST segment, QT interval, and T wave. When evaluating the QRS complex, the duration should be evaluated and the normal adult interval is 0.8-1.2 seconds with age variations. The duration may be prolonged in WPW, R BBB, and L BBB. The QRS amplitude may be elevated in LVH and RVH. ST segments should be evaluated for signs of ischemia. Different leads relate ischemia to different coronary distributions. The next aspect to approach is the QT interval, a normal QT interval is 0.4 seconds. The QT interval may be short in hypercalcemia, digitalis toxicity and congenitally shortened QT syndrome. There may be a prolonged QT interval in congenitally long QT syndrome, myocarditis, hypocalcemia, traumatic brain injury and with certain medications that cause prolonged QT interval. The last step is to evaluate the T wave morphology, see below for examples.
Dr. Hernandez competed her lecture with some clinical vignettes and a special thanks to Dale Dubin, MD for use of his pictures.
Matthew Jolley, MD (Children’s Hospital of Philadelphia) presented the lecture titled, “Practical Perioperative Transthoracic Echocardiography.” Dr. Jolley presented a few cases illustrating the clinical usage of perioperative transthoracic echocardiography (TTE). The first case was a five-year-old child with history of heart transplant in the emergency department (ED). He is ill, not keeping down immunosuppression x 2 days, has RLQ tenderness and needs help with decision making about going to the OR for an appendectomy for equivocal ultrasound. Your handy fellow grabs the TTE machine and places the probe in three locations to obtain some views for a quick quantitative evaluation of systolic function. The views your fellow obtained were an apical four chamber view, a parasternal short axis and a subxiphoid short axis view. To access qualitative systolic function there are two methods to use, fractional shortening (length based) and ejection fraction (volume based). Fractional shortening is the change in short axis ventricular diameter. A normal value for this would be greater than 28%.
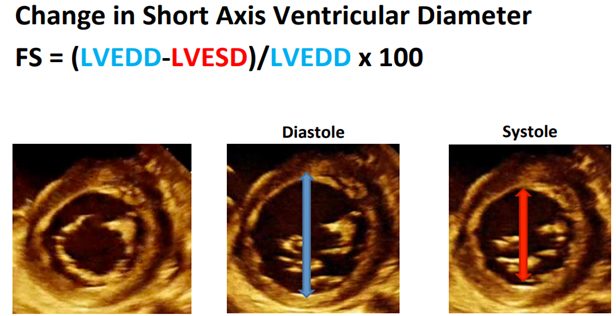
Measuring ejection fraction (EF) is a volume based method, using the equation EF = (LVEDV-LVESV)/LVEDV x 100, normal EF is >55%. Using Simpson’s method, we can estimate EF by measuring volume changes in the left ventricle.
The second case is a seven-year-old girl being evaluated for fainting episodes. Previously evaluated by neurology with no abnormalities, a screening EKG read as consistent with possible RVH and her cardiology appointment is still pending. She fainted chasing her brother up the stairs. Currently she is in the ED with a supracondylar fracture and there is concern for vascular compression. Your handy fellow again places the TTE probe and is able to get an estimation of right ventricular pressures using the Bernoulli equation (Pressure Difference (mmHg) = 4v2) with velocity in meters/second. You find that she has elevated pulmonary arterial pressures and bowing of the ventricular septum into the left ventricle. This gives you added information that you may need for planning of this urgent/emergent situation.
The last case is a 13-year-old male with a history of tetralogy of Fallot and severe asthma. He is one-month s/p a pulmonary valve replacement and presents to the emergency room with shortness of breath. You are called by the ED attending to intubate the patient. He is short of breath and doesn’t want to lie down. Again you have a TTE probe available and place it on his chest and find this:
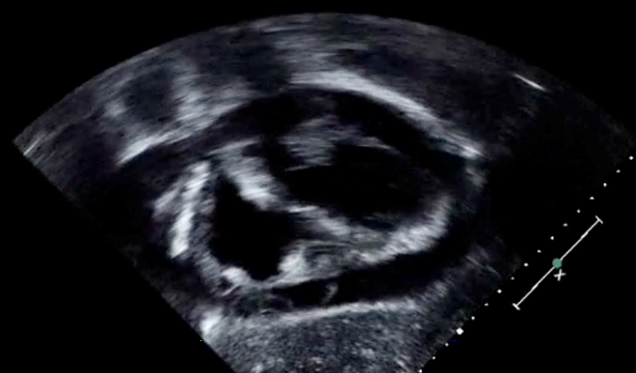
He has a large pericardial effusion and instead of getting intubated in the emergency room, you go to the cardiac catheterization lab for pericardial drain placement. The patient doesn’t need intubation and has improved ventilation, once the fluid is removed. TTE can provide valuable information, quickly. It does take time to become familiar with the views and how to perform them, but this information can be invaluable.
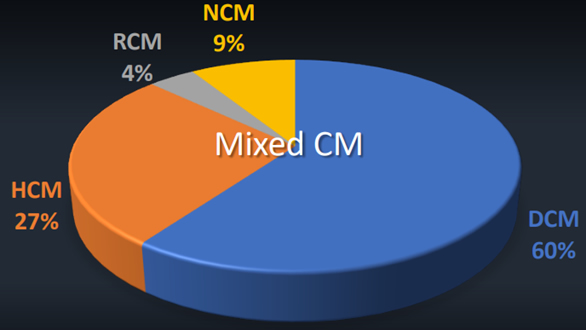
The last session of the cardiac refresher was “Anesthesia and Pediatric Cardiomyopathies,” delivered by Greg Latham, MD (Seattle Children’s Hospital). Dr. Latham had three learning objectives for his lecture; to understand the different cardiomyopathies (CM’s) in children, to develop a preoperative evaluation, and to be able to create an anesthetic plan for these patients. The World Health Organization defines cardiomyopathies as “Diseases of the myocardium associated with cardiac dysfunction.” There is an incidence of 1.5/100,000 children per year and 8/100,000 infants per year, while the overall prevalence remains unknown. It is a progressive disease and is the number one cause of heart transplant > 1 year-old. There has been an increase in the ability to identify the cause of the CM. In 2003 only 34% had an identified cause, this increased to 75% of cases by 2012. CM are divided into four classifications: dilated CM; hypertrophic CM; restrictive CM; and non-compaction CM.
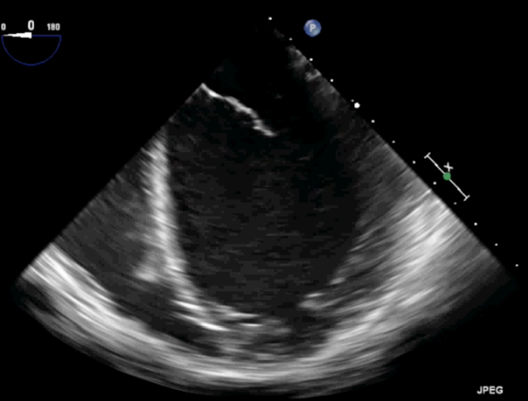
Dilated CM (DCM) is defined as “LV dilation with systolic dysfunction in the absence of valve disease, CHD, hypertension, or CAD.” There may or may not be RV dilation and dysfunction. It has an incidence of 0.6 cases/100,000 kids per year with increased rates in neonates. It has a heterogeneous etiology with myocarditis (35%-50%) being a large percentage mostly related to, parvovirus B19, coxsackie virus, adenovirus, influenza, Epstein–Barr, HIV, and herpes virus. The cause could also be genetic (>40%), toxic, neurohormonal, or idiopathic. Symptoms of heart failure vary depending on the age and severity of decreased function. The pathophysiology is a remodeling of the heart at the molecular, cellular and interstitial levels. There is dilation, fibrosis and thinning of the left ventricular walls. At presentation, 71% of patients will have heart failure and LV dysfunction, 54% will need intravenous inotropic support, 41% will need mechanical ventilation, 13% will need ECMO services, and 11% of patients will require urgent transplantation. Below is a classic echo picture of dilated cardiomyopathy:
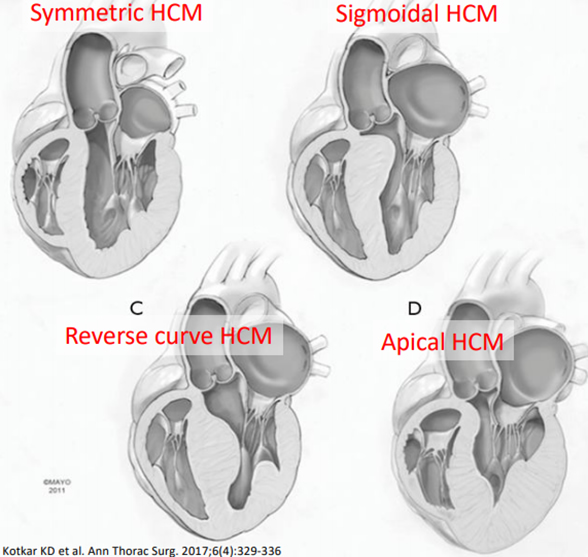
Hypertrophic cardiomyopathy (HCM) is defined as an “unexplained LV hypertrophy associated with non-dilated ventricular chambers in the absence of another cardiac or systemic disease” and the thickness is usually asymmetric. 60%-70% of HCM is genetic and related to Noonan’s syndrome, Pompe’s syndrome, muscular dystrophies, Friedreich ataxia, glycogen storage disease, and mitochondrial disorders. 36% of pediatric sudden cardiac death is related to HCM. There is an increased risk of death in the 1st year and then 1% mortality per year afterwards. Symptoms of HCM at presentation could range from asymptomatic to sudden cardiac death. The symptoms are based on: diastolic dysfunction; LVOTO; degree of MR; ↓CO; CHF; ischemia; and presence of arrhythmias. Heart failure is the number one cause of death. The presence of systolic anterior motion (SAM) of the mitral valve can cause decreased cardiac output. There are a variety of HCM’s:
Restrictive cardiomyopathy (RCM) is the least common and has the poorest prognosis. 80% of patients die or are transplanted within 5 years. Approximately 1/3 are of a mixed RCM/HCM etiology. Diastolic failure leads to heart failure with ↑ LVEDP, ↓ CO, ↑ LAP, resultant pulmonary hypertension, right heart failure, and arrhythmias with potential thrombosis.
Noncompaction cardiomyopathy (NCM) represents 5%-9% of pediatric CM’s. There is an embryologic arrest of myocardial development and often these represent a mixed phenotype. The symptoms are relative to the severity of LV dysfunction. There is an increased risk of sudden cardiac death, arrhythmias, thrombosis, and pulmonary hypertension. Heart transplantation is the only definitive cure.
Dr. Latham then proceeded to talk about the anesthetic risk of patients with cardiomyopathies. The POCA registry shows that 13% of the cardiac arrests were related to cardiomyopathy with a resultant 50% mortality. Kipps (2007) and Lynch (2011) both demonstrated increased complications and mortality with increased severity of systemic ventricular dysfunction. This leads to our preoperative evaluation. As anesthesiologists we should screen for unknown CM. This would be based on the family history, any syndromes associated with CM, evaluate for age appropriate cardiac reserve (any FTT, exercise capacity, keeping up with their peers), syncope, preoperative hypotension, any history of arrhythmias, or history of any cardiac murmurs. Patients with known CM, we should understand their phenotype and evaluate for any complications: thrombi, arrhythmia, pulmonary HTN.
This may require multidisciplinary planning with cardiology to ensure they are optimized for surgery. We should evaluate their medications such as: beta blockers, diuretics, ACE inhibitors. Are there any additional tests or laboratory studies required? Before the patient is taken to the operating room, their postop disposition should be discussed. There are also human, environmental, technical and logistical factors that should be accessed. Intraoperative management is based on the pathophysiology and disease severity.

Goals for dilated cardiomyopathy (DCM) under anesthesia are to keep the preload normal, afterload low to normal, heart rate normal to high, and contractility high with possible use of inotropes. Goals for hypertrophic cardiomyopathy (HCM) are to keep the preload normal to increased, afterload normal to high, heart rate low to normal with possible use of beta blockers to decrease the heart rate, and contractility normal with no use of inotropes. Restrictive cardiomyopathy (RCM) is treated similarity to cardiac tamponade or constrictive pericarditis. Under anesthesia, the preload goals would be normal to high, afterload should be kept normal, heart rate normal to high as stroke volume is fixed, contractility normal to high with the use of inotropes as needed and the pulmonary vascular resistance low.
Dr. Latham gave his TEN COMMANDMENTS OF CARDIOMYOPATHY; they are:
- Talk to the cardiologist
- Have a rescue plan
- Maintain cardiac output
- Maintain preload goals
- Maintain afterload goals
- Maintain oxygen supply/ demand
- Maintain a sinus rhythm
- Maintain heart rate goals
- HCM: Maintain SVR
- Titrate Anesthetics
There are many medications that we use in anesthesia that can be titrated slowly to effect. Keep in mind that propofol and volatile agents will decrease the patient’s SVR, while nitrous oxide, etomidate and opioids will keep it stable. Ketamine may have a stable to negative effect on contractility depending on the level of endogenous catecholamines. Last but not least important is to keep rescue medication close at hand. Medications that will increase the patient’s SVR are phenylephrine, norepinephrine, and vasopressin. Medications to increase contractility are milrinone, dobutamine, epinephrine, dopamine and ephedrine.






